The Saucy-Marbet reaction
26 November 2008 - named reactions
What: The Saucy-Marbet rearrangement

Who: G. Saucy & R. Marbet (Hoffmann-La Roche) 1967 (DOI)
Why: formation of a beta keto allene by reaction of a propargyl alcohol with an alkenyl ether by heat or acid catalysis.
Mechanism: the basic reaction mechanism is a variation of the Claisen rearrangement not with an alkene but with an alkyne.
Also known as: propargyl Claisen rearrangement. Actually preceded by earlier work by Black & Landor in 1965 (DOI)
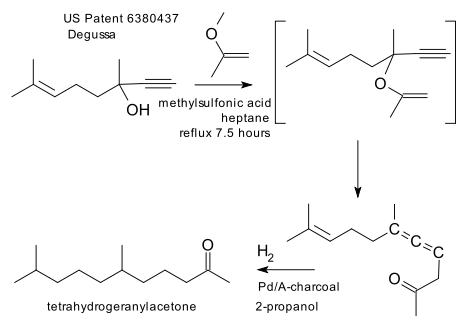
Industrial applications: synthesis of beta - ionone starting from Dehydrolinalool and ethyl acetoacetate (Link)
Scope: a 2002 patent (Link) describes synthesis of a geranyl acetone.
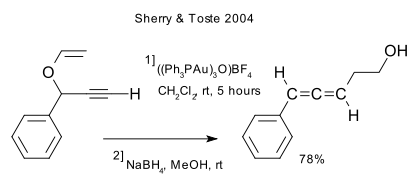
The reaction can be catalyzed by gold (DOI):
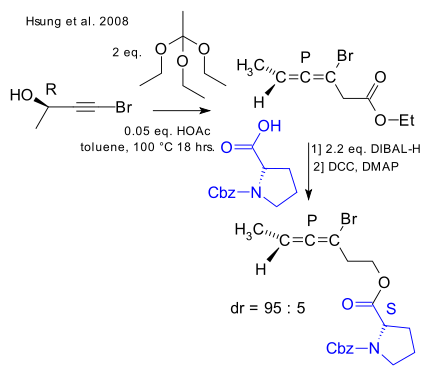
An example of a stereospecific reaction is provided by Hsung in 2008 (DOI):