Organogold part I
5 September 2008 - homogeneous catalysis Prehistory
Say organogold and you say gold catalysis, Organic chemistry's 21th century gold rush. Heterogeneous gold catalysis a well established concept but homogeneous catalysis was still pretty obscure 10 years ago. But is gold not supposed to be expensive and are there not a whole bunch of other metals out there that can do the required job just as well?
Lets take a look at some homogeneous gold catalysis prehistory. Ito in 1986 (DOI) reacted benzaldehyde and methyl isocyanoacetate with a chiral ferrocenylphosphine ligand and cationic gold(I) compound (Au(cHexCN)2)BF4 to form a chiral oxazoline. This reaction was a first in more than one way: asymmetric Aldol reactions already existed but this was also the first catalytic one.
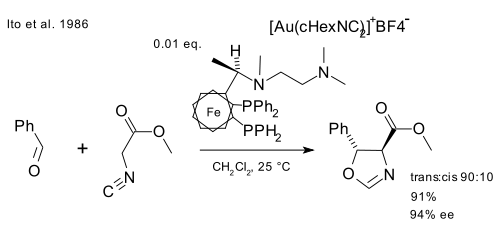
In a simple mechanistic picture, gold(I) simultaneously coordinates to two phosphine ligand and the carbon isocyanate group (Togni & Pastor 1990 DOI) which is then attacked by the carbonyl group.
In another development, Thomas in 1976 converted phenylacetylene to acetophenone using tetrachloroauric acid in a 37% yield (DOI). In this reaction gold(III) is used as a homogeneous catalyst replacing mercury in oxymercuration.
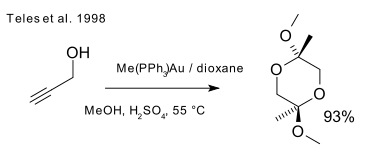
Utimoto in 1991 also tried out gold(III) (NaAuCl4) with alkynes and water (DOI). Teles identifies a major drawback of this method as Au(III) is rapidly reduced to catalytically dead metallic gold and returns to the theme of ligand supported Au(I) for the same transformation (DOI):
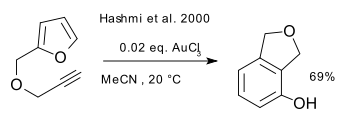
Another example of an Au(III) catalysed reaction is in a forced alkyne / furan Diels-Alder reaction (do not normally happen) ultimately forming a phenol (Hashmi et al. 2000 DOI):
This publication set of an avalanche of new homogeneous gold catalysis most notably in cycloisomerizations and cycloadditions.
Heterogeneous gold catalysis is an older science. Gold is an attractive metal to use because of its stability against oxidation and its variety in morphology for instance gold cluster materials. Gold has been shown to be effective in low-temperature CO oxidation and acetylene hydrochlorination to vinyl chlorides. The exact nature of the catalytic site in this type of process is hotly debated (2008 review (Hutchings et al. 2008 DOI).
The notion that gold can catalyse a reaction does not imply it is the only way. Too often many other metals can do the same job, notably in recent years cheap iron. In that respect in chemistry it appears iron is the new gold.
