Organogold part II
7 September 2008 - Fundamentals
Gold catalysis can be divided up into two main categories: heterogeneous catalysis including catalysts by gold nanoparticles (a whole hot research topic in itself) and homogeneous catalysis that can take place with gold(I) or gold(III) compounds. Common commercially available gold catalysts are gold(I) chloride, gold(III) chloride, chloroauric acid and a range of gold phosphines such as chloro(triphenylphosphine)gold(I). A popular co-catalyst combo is gold(III) chloride / silver triflate AgOTf (as if gold alone is not expensive enough)
Gold catalysis mainly effects compounds having alkene or alkyne bonds and does so in a type of bonding explained by the Dewar-Chatt-Duncanson model. Gold is certainly not the only metal showing this type of bonding and reactivity, several metal ions isolobal with the simple hydrogen proton (cationic: emptied s-orbital) do it as well: same-row elements mercury(II) and platinum(II). In 2007 Furstner & Davies (DOI) proposed the term pi acid as a placeholder for this type of ion (See also: cation-pi interaction).
The resulting metal-multiple bond complex is electrophilic in nature and activated for nucleophilic attack. In oxymercuration the metal part is played by mercury which is disadvantaged by a strong carbon-mercury bond which means stoichiometic use and requires an additional step to dissolve. A key advantage of gold is its labile Au-C bond which enables true catalysis. Other particular gold advantages are: air stability as high oxidation potential of Au(I) to Au(III) is a barrier to oxidation, carbophilic nature but not that oxophilic introducing tolerance towards water and alcohols, C-Au bonds that prefer hydrodeauration over beta-hydride elimination increasing reactivity and relative non-toxicity (Shen 2007 DOI) .
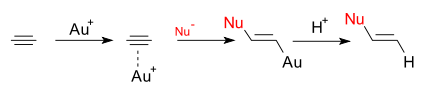
These advantages also set gold apart from the same-period elements silver and copper.
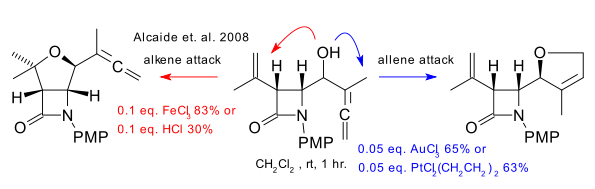
Gold is very chemoselective towards alkynes and allenes compared to alkenes. The example below (Alcaide et al. 2008 DOI) demonstrates the reverse can be true with other metals such as iron: