NNNS Chemistry blog
Prevous: Nobel Prize in Chemistry 2011 predictions
Next: The Quasi Crystal Nobel
Drunk Janus particles
02 October 2011 - Making It move VI
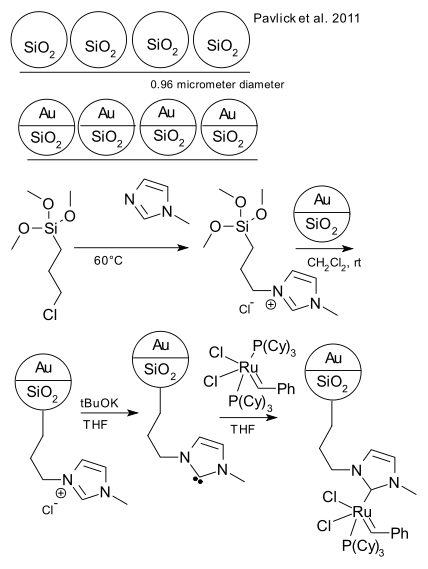 As part of our ongoing coverage of chemical making-it-move experiments, see part V, IV, III, II and I, now a brief summary of the recent attempt by Pavlick et al. at making a bead of silica move purposely in an organic solvent swimming pool (DOI). Here is the basic idea.
As part of our ongoing coverage of chemical making-it-move experiments, see part V, IV, III, II and I, now a brief summary of the recent attempt by Pavlick et al. at making a bead of silica move purposely in an organic solvent swimming pool (DOI). Here is the basic idea.
Take silica microspheres (see Stöber process), form a dense monolayer on a glass slide by pulling the slide through a biphasic system of hexanes and silica in ethanol and drying. Add a layer of chromium (7 nm) and then gold (56 nm) by sputter deposition. Free the spheres from the slide by sonication. The particles have now only one hemisphere covered by gold and hence are called Janus particles. Add a linker synthesised from 3-(chloro)propyl trimethoxy silane and 1-methylimidazole to the silica covered part and functionalise further with Grubbs' catalyst. Then set a microsphere loose in a trichloroethane solution of norbornene and track movement via digital video.
The spheres were found to move around at a speed of 0.30 micrometer per second and with some sense of direction. In one occasion a particle started of in a northbound direction then steered west and then headed south. The track was wobbly at best. A similar particle but without the gold treatment also moved around a lot but ended his random walk exactly where it had started.
The Grubbs' catalyst obviously converts norbornene to its polymer but only on one side of the particle. At the silica front monomer is depleted and a solvent flow starts up towards the back as a result of osmosis creating a motion against the flow. This proof of concept is for a change not all-play-and-no-work as Pavlick notes that in biology the bacterium listeria is known to propel itself by actin polymerization.
