Organogold part IV
13 September 2008 gold organyls
Chemical compounds containing a gold to carbon bond (organogold or gold organyls) exist with a wide range of structural motifs (Reviews: Herrmann, Elschenbroich, Parish, Patai). The gold carbide Au2C2 was discovered as early as 1900. The metal can exist as Gold(I) or gold(III).
Gold(I) complexes are 2-coordinate, linear , diamagnetic , 14 electron species. They exist as adducts LAuR with as ligand L for instance a triphenylphosphine or a isocyanide. The ligand prevents reduction of Au(I) to metallic Au(0) with dimerization of the organic residue. Gold(I) can also exist as aurate MAuR2 (the ate complex) whereby the cation is usually fitted with a complexing agent to improve stability. Gold is known to form acetylides (capable of forming polymeric structures), carbenes and carbynes. The classic method for the preparation of LAuR compounds is by reaction of a Grignard reagent with a gold(I) halide. A subsequent reaction with a organolithium R-Li forms the ate complex.
In a special group of compounds, an aryl carbon atom acts as a bridge between two gold atoms. One such compound, MesAu5,is formed in a reaction between Au(CO)Cl and the mesityl Grigard. Carbon can be coordinated with gold up to a value to 6. Compounds of the type C(AuL)4 are isolobal with methane and those of type C(AuL)5+ isolobal with the methanium ion.
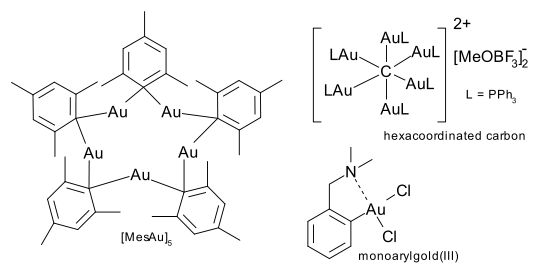
Gold(III) complexes are 4 coordinate , square planar, diamagnetic, toxic, 16 electron species. When the formal coordination number is less than 4, ligands such as chlorine can make up for it by forming a bridging ligand. Intramolecular chelation is another strategy. In general gold(III) compounds are toxic and therefore less studied than gold(I). For all practical purposes the chemistry is confined to monoarylgold(III) complexes.
