metal-free or almost-no-metal reactions
15 February 2009 - news
An alkyne hydration catalysed by a gold/silver system at a 10 ppm level with turnover number reaching 84,000 has recently been reported (Marion et al. DOI).
 .
.
In this system the ligand is a N-heterocyclic Carbene. At the same time, reports on no-metal at all (metal-free) organic reactions are abundant which on the one hand seems even better but on the other makes you wonder if in these systems minute quantities of catalyst present are not simply overlooked.
Lets take some reports from the recent literature and check for analytical chemistry on metal trace amounts, and while we are at it, check presence of a control experiment (what happens if metal IS present), check for mechanism (how does the reaction work when metal is omitted), test for scope, test for atom economy and finally, check for efficiency (is the uncatalysed reaction worth the wait)
The metal-free Ferrier rearrangement presented by De et al. (DOI) is not truly a metal-free reaction because the element boron in boron trifluoride that is replaced by hexafluoropropanol (HFIP) is not a metal but a metalloid. On the upside, a mechanism as to why HFIP works just as well is provided.

In a metal-free Trost allylic alkylation, recently reported by Cheng et al. (DOI), palladium is replaced by (stoichiometric) oxidizing reagent DDQ . Key step in the reaction mechanism is formation of a charge-transfer complex.
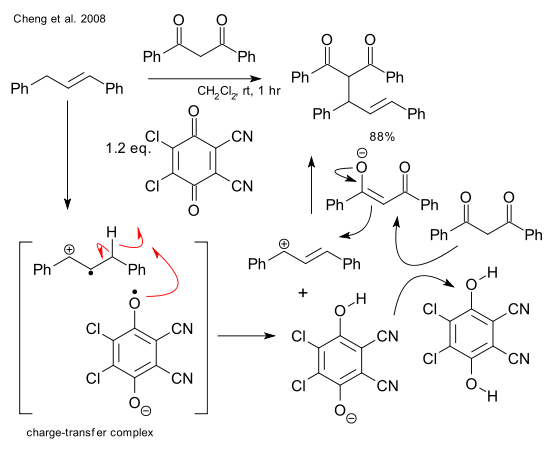
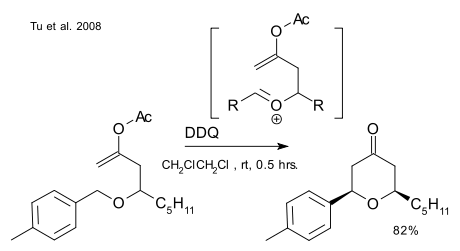
A report by Tu et al. (DOI) describing a Prins-like reaction with very similar DDQ chemistry (and published only a month or so prior to the one above) does not bother to specify the metal being replaced:
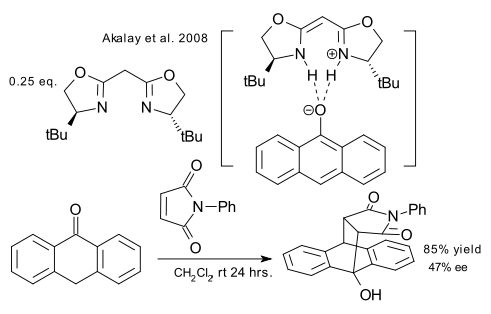
Finally, a metal-free asymmetric BOX ligand is employed in the Diels-Alder reaction of anthrone with a maleimide (Akalay et al. DOI). A metal is usually required to anchor the ligand to the substrate but this report shows that hydrogen bonding will do as well.
What do the above examples have in common? No surprises there: lack of control experiments, need for specific substituents for the reaction to work, no hunt for hidden metals, not always clear what metal is being replaced. On the upside all reports are accompanied by handsome reaction mechanisms.
