Twisted amides
16 March 2009 - News
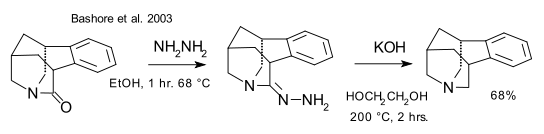
A new twisted amide has been described recently by Szostack et al. (DOI) with a twist angle of 60° and stable in water. The final step of its synthesis is a Schmidt reaction just like that other twisted amide 2-quinuclidone.
In regular amides the carbonyl group and the amine group share the same plane due to partial double bond character of the C-N bond caused by delocalization of the amine lone pair into the carbonyl oxygen. Hence, the carbonyl group does not behave as such and the amine group lacks basicity. Except when you introduce twisting.
This tweak is relevant to biochemistry : twisted amides may help explain cis-trans isomerization of amides in protein folding and amide hydrolysis by peptidase in other biochemical processes as already observed by Somayaji & Brown in 1986 (DOI).
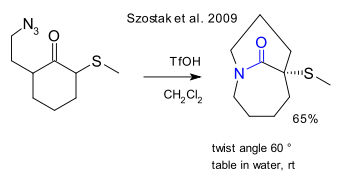
In 2001 Kirby et al. described an adamantane based twisted amide with a larger twist angle of 90° (DOI). This compound shows rapid hydrolysis and its carbonyl group has Wittig reaction capabilities.
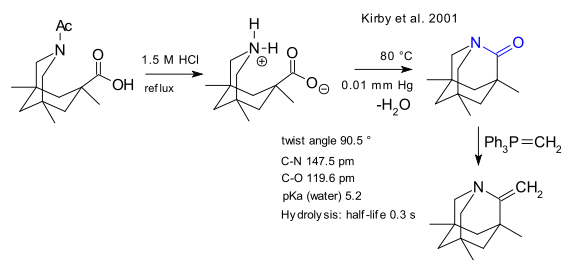
Another amide is reduced via Wolff-Kishner reduction to a tertiary amine (Bashor et al. 2003 DOI)