The 12-step cascade
07 January 2012 - Chemistry records
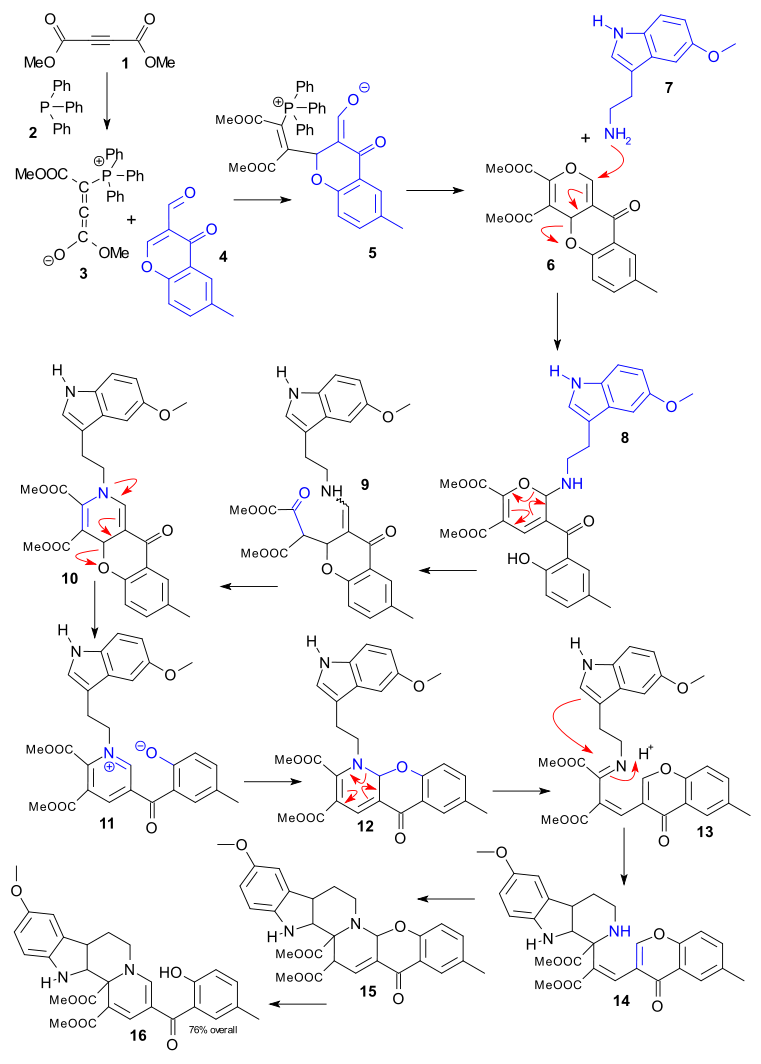 A large Germany-based team (Stefan Grimme of FLP fame among them) report a record 12-step cascade reaction (Dückert et al. 2011 DOI) in their quest for novel (medicinal?) indoloquinolizines. They like to call their effort biology-oriented synthesis because nature just loves cascade reactions in the construction of really large and complex molecules. The reaction takes place in a single vessel and starts off with three reagents. At a later stage a fourth and fifth are added which, considering the definition of a cascade reaction (all-in-one intramolecular reaction) technically makes it a combined 4 and 8-step cascade.
A large Germany-based team (Stefan Grimme of FLP fame among them) report a record 12-step cascade reaction (Dückert et al. 2011 DOI) in their quest for novel (medicinal?) indoloquinolizines. They like to call their effort biology-oriented synthesis because nature just loves cascade reactions in the construction of really large and complex molecules. The reaction takes place in a single vessel and starts off with three reagents. At a later stage a fourth and fifth are added which, considering the definition of a cascade reaction (all-in-one intramolecular reaction) technically makes it a combined 4 and 8-step cascade.
The 12-steps combined takes us through a whole catalogue of reaction mechanisms and here we go: Dimethyl acetylenedicarboxylate 1 reacts with triphenylphosphine 2 to zwitterion 3 which then reacts with chromone 4 to adduct 5 by alkylation. Phosphine is eliminated and the ring is closed in 6, amino indole 7 and camphorsulfonic acid are then added forming 8 in a conjugate N-alkylation, the pyran opens and O addition gives intermediate 9, amine-carbonyl condensation gives dihydropyridine 10, ring opening and Pyridinium rearomatisation gives 11, nucleophilic aromatic addition of oxygen gives 12, an aza-claisen rearrangement gives 13, a Pictet-Spengler reaction gives 14,an aza-michael addition gives 15 and finally a retro-Michael addition gives 16.
The reaction takes up to 30 minutes with an overall 76% yield.
Also see: The 8-component reaction
