Target : Lysergic acid
16 March 2011 - Pd power
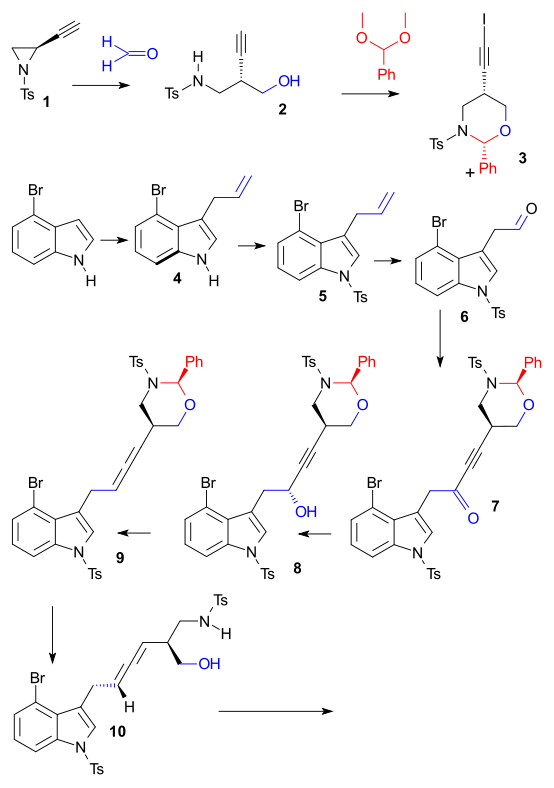 Lysergic acid is a biomolecule and precursor to many alkaloids. Derivatives are used as pharmacauticals, the notorious lysergic acid diethylamide one of them. Inuki et al. do not state an urgent reason for recreating the compound in the laboratory other than showing of some new chemistry, more specifically palladium-catalyzed cyclizations of allenes DOI. Tosyl protected Chiral 2-ethynylaziridine 1 (obtained from L-serine) reacted in a reductive coupling with formaldehyde to alcohol 2 (Tetrakis(triphenylphosphine)palladium(0), InI). Protection of the amino alcohol with benzylidene acetal (CSA) and (silver nitrate, NIS) then gave iodo-alkyne 3. Starting point for building block number two was 4-bromoindole which was reacted with allyl alcohol to alkene 4 (Tetrakis(triphenylphosphine)palladium(0), triethyl borane), then with tosyl chloride (protection) to N-tosyl 5. Oxidative cleavage (OsO4
Lysergic acid is a biomolecule and precursor to many alkaloids. Derivatives are used as pharmacauticals, the notorious lysergic acid diethylamide one of them. Inuki et al. do not state an urgent reason for recreating the compound in the laboratory other than showing of some new chemistry, more specifically palladium-catalyzed cyclizations of allenes DOI. Tosyl protected Chiral 2-ethynylaziridine 1 (obtained from L-serine) reacted in a reductive coupling with formaldehyde to alcohol 2 (Tetrakis(triphenylphosphine)palladium(0), InI). Protection of the amino alcohol with benzylidene acetal (CSA) and (silver nitrate, NIS) then gave iodo-alkyne 3. Starting point for building block number two was 4-bromoindole which was reacted with allyl alcohol to alkene 4 (Tetrakis(triphenylphosphine)palladium(0), triethyl borane), then with tosyl chloride (protection) to N-tosyl 5. Oxidative cleavage (OsO4
/ NaIO4) gave aldehyde 6. Chunks 6 and 3 were then joined in a Nozaki-Hiyama-Kishi reaction followed by alcohol oxidation to ketone 7 using Dess-Martin periodinane and asymmetric reduction back to an alcohol but now chiral using Alpine borane. The allene 8 was formed next with nosyl hydrazine / Mitsunobu reaction conditions.
The key transformation was a complex Pd catalyzed domino reaction (Tetrakis(triphenylphosphine)palladium(0) / potassium carbonate) to tetracycle 14 involving an oxidative addition, aminopalladation, reductive elimination. The final reactions consisted of alcohol oxidation (Dess-Martin periodinane) and esterification (Trimethylsilyldiazomethane) to ester 14, followed by detosylation (Sodium naphthalenide) - sequence spoiled by epimerisation - and methylation (formaldehyde / sodium borohydride) to 15 and then reduction to lysergic acid (lithium aluminium hydride) 16
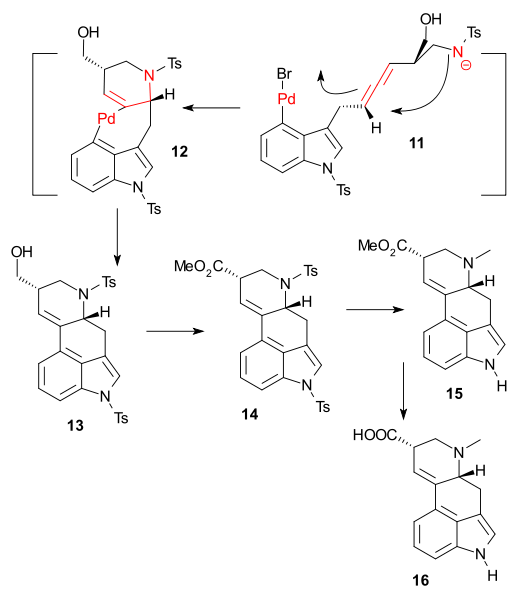
Destination reached! Palladium count: 3.
