NNNS Chemistry blog
Prevous: The mystery of the missing methane part II
Next: Retire hybrid atomic orbitals? Not yet!
Target: (+)-mefloquine
10 June 2011 - Total synthesis
 Mefloquine hydrochloride a.k.a. Lariam is an important antimalarial drug (quinine analogue). It is sold as a racemate but uncertainty exists about the effectiveness of the individual enantiomers (R,S) and (S,R). Adding a long list of clinical side effect , an investigation is in order. Knight, Sauer & Coltart did their bit and have reported a new asymmetric synthesis for the compound (DOI).
Mefloquine hydrochloride a.k.a. Lariam is an important antimalarial drug (quinine analogue). It is sold as a racemate but uncertainty exists about the effectiveness of the individual enantiomers (R,S) and (S,R). Adding a long list of clinical side effect , an investigation is in order. Knight, Sauer & Coltart did their bit and have reported a new asymmetric synthesis for the compound (DOI).
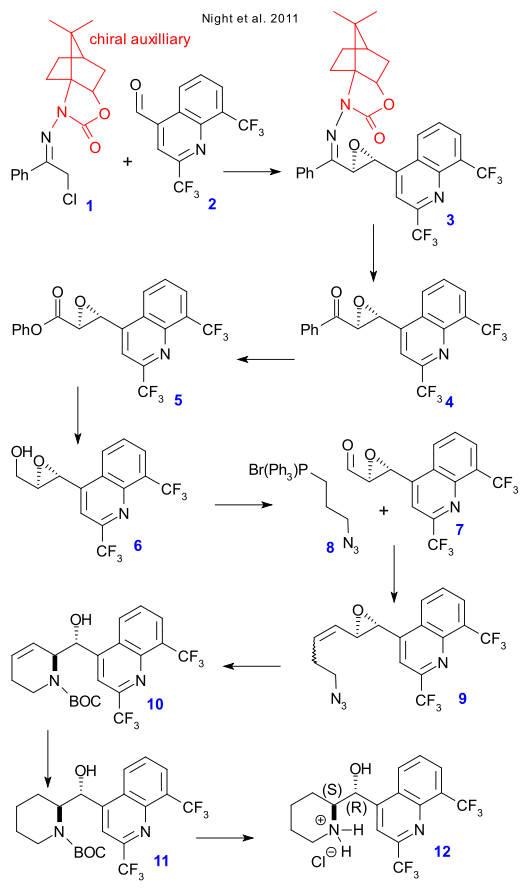
Step 1 is a Darzens reaction of hydrazone 1 with ketone 2 forming chiral epoxide 3 thanks to the presence of an chiral auxiliary on 1. This auxiliary was then removed to form ketone 4 (TsOH), a Baeyer-Villiger oxidation (m-CPBA) then formed ester 5, ester reduction (lithium aluminum hydride) gave alcohol 6 and then oxidation via the Dess-Martin periodinane gave aldehyde 7.
Wittig reaction with ylide 8 then gave alkene 9, reaction with triphenylphosphine gave a Staudinger reaction to the amine and in-situ reaction with the epoxy group then gave the N-BOC alcohol 10 after protection with di-tert-butyl dicarbonate. The alkene group was reduced (H2, Pd-alumina) to 11 and the BOC group was then removed using trifluoroacetic acid and after adding HCl mefloquine hydrochloride 11 was obtained.
