NNNS Chemistry blog
Prevous: Novel silicon based fragrances
Next: Silver particles, molecules, ions?
Tackling oil sands
23 July 2010 - Petrochemistry
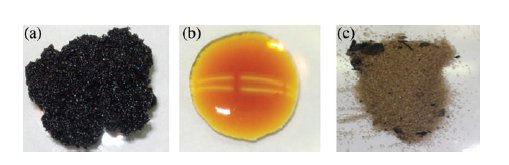 The black goo on the left in the picture is crude Alberta oil sand, an ugly mixture of sand, clay, bitumen and water. Present-day isolation of the bitumen (crude oil!) requires an equally ugly process: hot-water extraction to get rid of the sand, coking at plus 500 °C followed by catalytic hydrocracking to get lighter fractions and catalytic hydroprocessing (300-500 °C, 18MPa H2) to get rid of heteroatoms and metals. The overall process requires a lot of energy, water and produces a lot of waste.
The black goo on the left in the picture is crude Alberta oil sand, an ugly mixture of sand, clay, bitumen and water. Present-day isolation of the bitumen (crude oil!) requires an equally ugly process: hot-water extraction to get rid of the sand, coking at plus 500 °C followed by catalytic hydrocracking to get lighter fractions and catalytic hydroprocessing (300-500 °C, 18MPa H2) to get rid of heteroatoms and metals. The overall process requires a lot of energy, water and produces a lot of waste.
Brough at al. (DOI) in a new publication report an alternative method using supercritical carbon dioxide. Oil sand, toluene and a catalyst (Rh/C, Ru/C) are mixed in a high pressure vessel at 100°C and hydrogen (6 MPa) and carbon dioxide (12MPa) are added. In the resulting petroleum product the API gravity (density of a petroleum liquid compared to water) has increased from 8 to 16° (the Wikipedia article will explain why the unit is a degree....), sulfur and metal (Ni, V) content has decreased and H/C ratio has increased. The researchers are quite pleased with themselves: the yellow substance in the central picture looks like a regular oil and the with the sand in image three you can almost start a beach.
