Superbase superacid
07 February 2014 - Chemistry book of world records
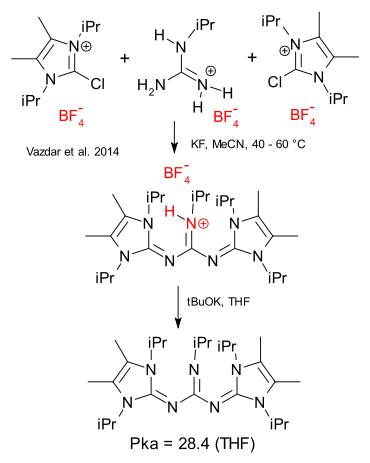 Two chemistry world records in rapid succession. Record one: a superbase as reported by Vadzar here. Superbases are nothing new, examples are TMG with an pKa of the conjugated acid of 17, phosphazene base tBuP1(pyrr) (20,2), triazabicyclodecene (21.7) and a guanidino-phosphazene hybrid with pKa 27. The new superbase is synthesised from an imidazolium salt and a guanidine with a record pKa of 28.4. The compound derives it's basicity from three factors: on protonation it has an aromatization gain, steric strain is relieved and as added bonus the sheer size of the base makes it easier for the positive charge to disperse.
Two chemistry world records in rapid succession. Record one: a superbase as reported by Vadzar here. Superbases are nothing new, examples are TMG with an pKa of the conjugated acid of 17, phosphazene base tBuP1(pyrr) (20,2), triazabicyclodecene (21.7) and a guanidino-phosphazene hybrid with pKa 27. The new superbase is synthesised from an imidazolium salt and a guanidine with a record pKa of 28.4. The compound derives it's basicity from three factors: on protonation it has an aromatization gain, steric strain is relieved and as added bonus the sheer size of the base makes it easier for the positive charge to disperse.
There is also news from the superacid front. Superacids are nothing new, examples are fluoroantimonic acid with an pKa of -25 and the carborane acid H(CHB11Cl11) (do mind that chemists love to confuse their audiences with different acidity definitions and that measurement rely on the collaboration of solvents and temperature windows). The people that brought you the carborane acid now moved on to H(CHB11F11) with chlorine replaced by fluorine (DOI). The compound was synthesised by reacting Cs(CHB11H11) first with hydrogen fluoride and then with 20 bar of fluorine gas, a procedure that was repeated 5 times. A practical procedure was difficult to establish because the reaction product as it turned out reacts with just about any hydrocarbon. The procedure is also not without risks to personal well being, the experimental info mentions "full-body protective clothing and the buddy system" which probably means that in the event of an explosion or gas leak two deaths will occur instead of just one. The compound reacts with benzene to a benzenium ion (like the chlorine counterpart) but it protonates just as easily n-hexane forming a carbocationic salt and hydrogen gas (unlike the chlorine counterpart).
