NNNS Chemistry blog
Prevous: Reversing the Pinacol coupling
Next: Meanwhile in the Whitesides lab (IV)
Stereoinversion at tertiary carbon
21 September 2013 - Orgo
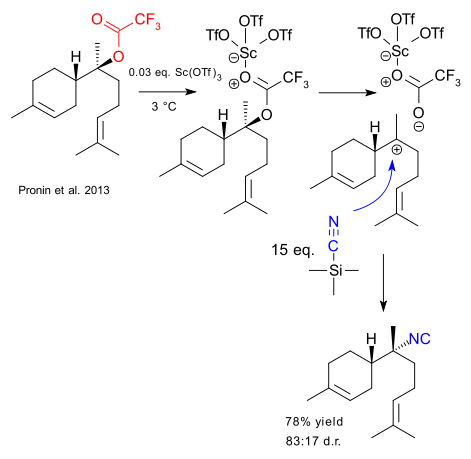 Is nothing sacred anymore? Earlier this year it was reported that you do not really need crystals for crystallography (although the initial enthusiasm seems to have cooled down a bit) and now we have a report here by Pronin et al. that a substitution reaction on tertiary carbon can be stereoselective as in SN2. To quote Wikipedia: ' tertiary substrates do not participate in SN2 reactions, because of steric hindrance. ' and yet Pronin found a loophole. The substrate in his proof of concept is an tert-alkyl trifluoroacetate, the Lewis acid catalyst is scandium triflate , the nucleophile / solvent is trimethylsilylcyanide (TMSCN) and the product is an isocyanide. The rationale for the observed stereoinversion is the formation of an intimate ion pair.
Is nothing sacred anymore? Earlier this year it was reported that you do not really need crystals for crystallography (although the initial enthusiasm seems to have cooled down a bit) and now we have a report here by Pronin et al. that a substitution reaction on tertiary carbon can be stereoselective as in SN2. To quote Wikipedia: ' tertiary substrates do not participate in SN2 reactions, because of steric hindrance. ' and yet Pronin found a loophole. The substrate in his proof of concept is an tert-alkyl trifluoroacetate, the Lewis acid catalyst is scandium triflate , the nucleophile / solvent is trimethylsilylcyanide (TMSCN) and the product is an isocyanide. The rationale for the observed stereoinversion is the formation of an intimate ion pair.
So far so good. The difficult part as always is the prior art. No citation is given for any article presenting stereoinversion at any tertiary carbon. On the other hand a citation is given for a reaction of a tertiary halide with TMSCN (dating back to 1981) but not with stereoinversion and one citation is given for a stereocontrolled isocyanation of an alcohol (2006) but by a different reaction type.
Another difficult part is the reaction mechanism. The interesting bit is that the reaction apparently only works with the nucleophile as solvent e.g. in large excess. The complete solubility of scandium triflate in TMSCN also has to be accounted for. How can this be explained? In the mechanism presented here the Lewis acid activates the carbonyl group in a well-established manner. Pronin et al take a different view: they suspect the presence of something like Sc(TMSCN)n(OTf)3 and the larger steric bulk would indeed favor the intimate ion pair mechanism. On the other hand the proposal has no literature precedent and the article does not even provide a hint. Of course, the reaction is expected to be very slow so a large excess of nucleoplhile makes all the difference.
