NNNS Chemistry blog
Prevous: Replaced by computational overlords not just yet
Next: The new thermite reaction
Sanofi artemisinin update
17 June 2015 - Drug research
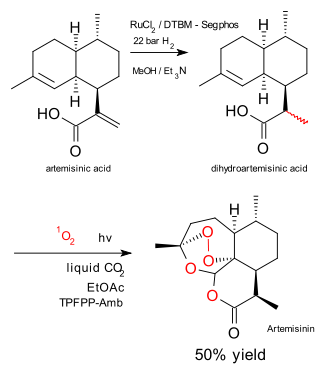 The people at Sanofi continue to tinker away at their industrial production of artemisinin. As described in a previous blog episode here the starting compound artemisinic acid (produced from glucose) was converted to artemisinin in subsequently a hydrogenation, an esterification and an Schenk-ene step with one equivalent of singlet oxygen. In new research described here (Nature Chemistry, Zamara et al.) the Sanofi group and partners demonstrate that the esterification step can be done away with and replaced by a direct Schenck ene oxidation.
The people at Sanofi continue to tinker away at their industrial production of artemisinin. As described in a previous blog episode here the starting compound artemisinic acid (produced from glucose) was converted to artemisinin in subsequently a hydrogenation, an esterification and an Schenk-ene step with one equivalent of singlet oxygen. In new research described here (Nature Chemistry, Zamara et al.) the Sanofi group and partners demonstrate that the esterification step can be done away with and replaced by a direct Schenck ene oxidation.
The new approach is a lot greener. The new features are: liquid carbon dioxide solvent (not the same as supercritical carbon dioxide!), ethyl acetate co-solvent, a solid porphyrin / amberlyst catalyst and a continuous-flow photochemical reactor. Reported yield 50% at 90% conversion.
Just for how long are photochemical reactions in industry considered of just great potential? The authors cite a G. Ciamician as a true believer. Problem is the guy wrote about it in 1912.
