SOMO catalysis
22 December 2008 - Concepts
SOMO catalysis. Introduced by W.C. MacMillan in 2007 as an extension of two other concepts in chiral amine based organocatalysis : HOMO activation involving 4 pi electrons in enamine catalysis (increased HOMO energy activates a reaction) and LUMO activation with 2 pi electrons of iminium ions (decreased LUMO energy activates reaction). In SOMO activation a radical cation with an activated SOMO molecular orbital is formed by one-electron reduction of an enamine complex.

In a first report an aldehyde reacts enantioselectively with an allyltrimethylsilane as SOMO nucleophile (or somophile) in presence of a second-generation MacMillan organocatalyst and oxidizing reagent CAN (Beeson et al. DOI)
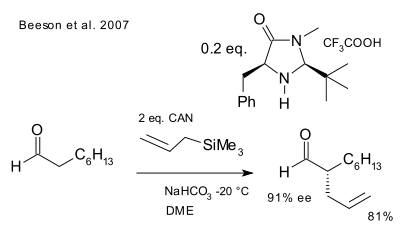
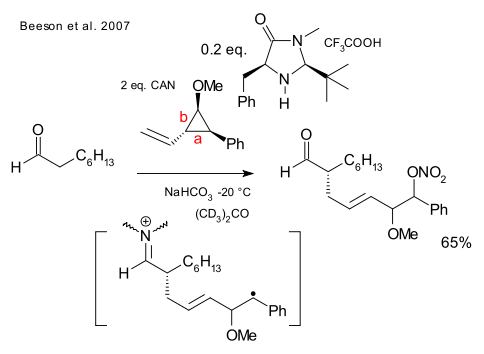
A reaction with a cyclopropane radical clock demonstrates that the intermediate truly is a radical because the ring opens along bond a forming a stable benzyl radical and not along bond b where a stabilized oxonium ion would expose a cationic intermediate.
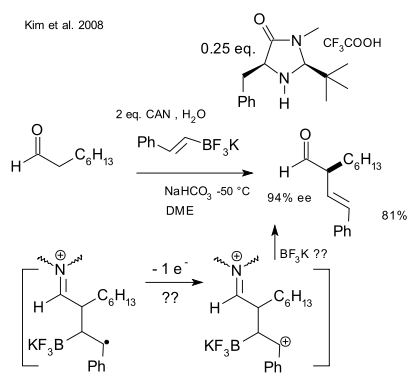
In a recent extension a vinyl potassium trifluoroborate salt was thrown in resulting in a alpha vinyl aldehyde (Kim et al. 2008 DOI) although this blog struggles to understand the dicationic intermediate and the discrete molecule of BF3K expelled for this reaction to work, at least according to the investigators on duty.
Styrene is also found to react with aldehydes in this methodology with the intermediate radical neutralized (after another one-electron reduction to the carbocation ion) by a nitrite ion (Graham et al. DOI).
Reactions of enamine radical cations (as a reactant, not as a catalyst) were first described by Narasaka et al. in 1992 (DOI):

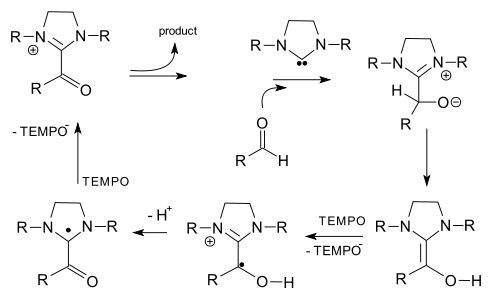
The SOMO organocatalysis concept has not yet attracted support from other research groups other than that of MacMillan himself except perhaps that of the Grimme group as evidenced by a recent 2008 report on aldehyde oxidation in a NHC / TEMPO combo (Quin et al. DOI):
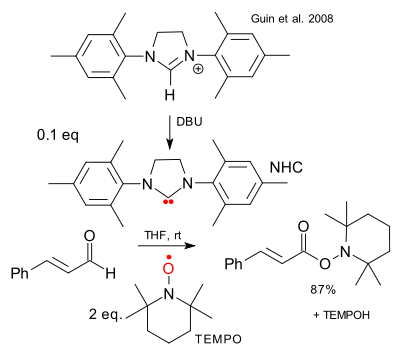
with this fancy mechanism: