Removable directing groups
18 December 2010 - Org. Synth. Strategy
 Regioselectivity in aromatic electrophilic substitution continues to attract chemical research even though the reaction is over 100 years old. The problem is this regioselectivity can be difficult to achieve. Take a monosubstituted arene, throw a chemical at it and see where it ends up. In a totally unspecific reaction the new substituent can occupy one of 3 different positions (ortho, meta or para) and with multiple substitutions the mix gets even more complicated. Regioselectivity can be introduced by so-called directing groups. Activating groups promote ortho and meta substitution and deactivating groups that of meta substitution. But what to do with them when the reaction is done?. An old trick that gets rediscovered from time to time is the use of a removable directing group. A classic is the sulfonic acid group in aromatic sulfonation or the carboxyl group. Recent case studies involving removable directing group are those on pyridyl silyl groups DOI, carboxyl groups DOI and cyano groups DOI.
Regioselectivity in aromatic electrophilic substitution continues to attract chemical research even though the reaction is over 100 years old. The problem is this regioselectivity can be difficult to achieve. Take a monosubstituted arene, throw a chemical at it and see where it ends up. In a totally unspecific reaction the new substituent can occupy one of 3 different positions (ortho, meta or para) and with multiple substitutions the mix gets even more complicated. Regioselectivity can be introduced by so-called directing groups. Activating groups promote ortho and meta substitution and deactivating groups that of meta substitution. But what to do with them when the reaction is done?. An old trick that gets rediscovered from time to time is the use of a removable directing group. A classic is the sulfonic acid group in aromatic sulfonation or the carboxyl group. Recent case studies involving removable directing group are those on pyridyl silyl groups DOI, carboxyl groups DOI and cyano groups DOI.
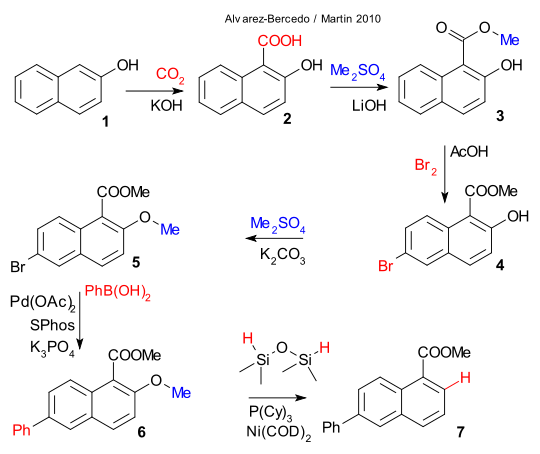
In a most recent exploit Alvarez-Bercedo and Martin have investigated the aryl ether group as a potential removable directing group (DOI). The structure 7 in the scheme below is a naphthalene with a carboxylic ester group at position 1 and a phenyl group at position 5. Apparently raw materials 1-carboxylnaphthalene or 3-phenylnaphthalene have the wrong directing groups at the wrong places so a cunning plan is required that features 2-naphthol 1. A sequence of reaction steps then takes place creating the desired substituents as follows: carboxylation to 2 (carbon dioxide/potassium hydroxide), esterification to 3 (dimethyl sulfate / lithium hydroxide), bromination to 4 (bromine / acetic acid), alkylation to 5 (dimethyl sulfate / potassium carbonate) and phenyl Suzuki coupling to 6 (phenylboronic acid / palladium acetate / SPhos / tripotassium phosphate).
In the final surprise step the methoxy group has played its role and is removed by dimethyldisoloxane as hydrogen donor and Nickel bis(cyclooctadiene) / tricyclohexylphosphine as catalyst. As an encore Alvarez-Bercedo and Martin and prooved the silicon compound truly is the hydrogen donor by applying a deuterated one.
