Properties along a gradient
24 October 2011 - Physical chemistry
 This blog is the proud owner of a 500 ml volumetric flask filled to the rim with PET granules, a souvenir from a botched career in plastics recycling. Should the flask ever become the topic of scrutiny in a chemical laboratory, the lab technicians may be surprised to find that the melt viscosity of the granules increase going from the bottom of the flask to the top. Pretty irrelevant of course, the flask was filled with PET samples in order of viscosity for no reason at all.
This blog is the proud owner of a 500 ml volumetric flask filled to the rim with PET granules, a souvenir from a botched career in plastics recycling. Should the flask ever become the topic of scrutiny in a chemical laboratory, the lab technicians may be surprised to find that the melt viscosity of the granules increase going from the bottom of the flask to the top. Pretty irrelevant of course, the flask was filled with PET samples in order of viscosity for no reason at all.
Functionally graded materials on the other hand are well known in industry and are often based on composites with varying composition along a gradient. Moving on to the micro- and nanoscale, gradient materials are an active research field. Imagine a surface that is superhydrophobic on one end and smoothly becomes superhydrophilic when going to the other end. The surface gradient material demonstrated by Yu in 2006 (DOI) is based on a gold layer covered by gold clusters by electrodeposition. This surface is very rough and therefore very hydrophobic (see Cassie's law). Hydrophobicity is further increased by covering the surface with a monolayer a long aliphatic thiol (ethanol solution dip) and because the surface coverage is related to immersion time a gradient presents itself. The unoccupied positions are then filled by a thioalcohol again by dipping making the surface increasingly hydrophilic. In similar configurations a water droplet set loose on a gradient surface will be able to propel itself to the other side.
Goldflam et al. have recently demonstrated a gradient in a so-called split-ring resonator used in negative refractive index research(DOI). The SSR is built on a sapphire layer from vanadium oxide by lithography. In the far-infrared an absorption maximum is evident regardless of the position on the layer. However when a voltage is applied each spot on the layer now has its own absorption characteristics. The cause is unclear but it has to do with local heating inducing a vanadium oxide phase transition from an insulator to a metal. The link with refraction is not directly obvious.
Ahmed et al. let gravity to all the work (DOI). They are into the gradient porous material business and mention nature's engineering skills in bone biosynthesis. Their recipe for gradient porous material starts with an oil-in-water emulsion made from cyclohexane and aqueous polyvinyl alcohol. This emulsion is centrifuged and rapidly cooled and then solvent is removed. The material can be fortified by adding silica particles (Stöber process) which travel in the opposite direction as the oil droplets.
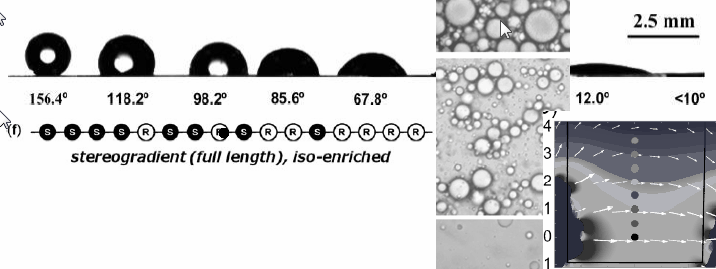
We can even narrow our view to the molecular level to look for gradients, for example in gradient copolymers. In one demonstration Nakamo et al. polymerise racemic propylene oxide to polypropylene carbonate using a special chiral catalyst (DOI). This catalyst converts the (S) monomer twice as fast as the (R) monomer making the chain a stereogradient starting completely isotactic and then gradually becoming isotactic. Turning this copolymer into a gradient material is another challenge and then finding something useful to do with it an even bigger one. One surprising property is higher thermal decomposition temperature compared to either enantiomeric polymers of the stereoblock copolymer: something to do with defeating intramolecular stereocomplex formation.
