NNNS Chemistry blog
Prevous: It is easy being a global warming skeptic
Next: The double dehydro-Diels-Alder Reaction
Phenol hydrogenation
30 November 2009 - catalysis
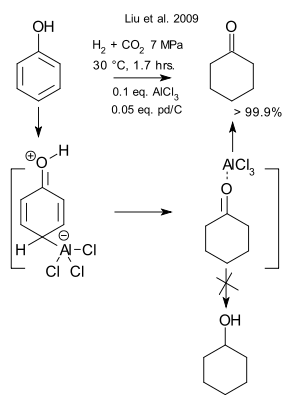 Cyclohexanone is industrially made by cyclohexane oxidation. it can also be made from cheaper phenol by hydrogenation but then spillover to cyclohexanol is just around the corner. In a recent Chinese publications this issue is tackled by combining a catalyst - palladium on carbon - with a Lewis acid - aluminium chloride in a phenol / scCO2 system (Liu et al. 2009 DOI). No cyclohexanol is formed and the authors offer a simple explanation. The Lewis acid is expected to activate the aromatic ring (just as in EAS) and palladium activates hydrogen. The carbon dioxide in addition to being a solvent speeds up the reaction even further by being a weak Lewis acid itself. Finally, By complexation with cyclohexanone the Lewis acid blocks the secondary reaction to the alcohol.
Cyclohexanone is industrially made by cyclohexane oxidation. it can also be made from cheaper phenol by hydrogenation but then spillover to cyclohexanol is just around the corner. In a recent Chinese publications this issue is tackled by combining a catalyst - palladium on carbon - with a Lewis acid - aluminium chloride in a phenol / scCO2 system (Liu et al. 2009 DOI). No cyclohexanol is formed and the authors offer a simple explanation. The Lewis acid is expected to activate the aromatic ring (just as in EAS) and palladium activates hydrogen. The carbon dioxide in addition to being a solvent speeds up the reaction even further by being a weak Lewis acid itself. Finally, By complexation with cyclohexanone the Lewis acid blocks the secondary reaction to the alcohol.
By one of those nice coincidences this article was dropped in the mailbox of one publisher the very same day (7 July 2009) another article also on cyclohexanone hydrogenation (Chatterjee et al. 2009 DOI) appeared online with another publisher. The solvent is again scCO2 and the catalyst is palladium embedded in mesoporous silica that is doped with sodium aluminate forming Pd/Al-MCM-41. At 50°C and 12GPa (H2 + CO2) the results are comparable. The presence of a Lewis acid is not appearant but the aluminium incorporated in the silica is creating acidic sites just the same.
