Phenanthrene H-H bond revisited
21 March 2009 - physical organic chemistry
Phenanthrene is a peculiar molecule that chemists are unable to cope with. The two bay hydrogen atoms are in very close proximity to each other which is prompting one group to say their interaction must be repulsive and another group to say that this interaction must be a result of bonding as in a hydrogen-hydrogen bond. The opponents in this longstanding debate do their arguing mostly through computer models but now Grimme, Erker and coworkers, in a recent article (DOI), have summoned a new powerful weapon called Experimental Evidence. As a result, they now demand the complete surrender of this hydrogen-hydrogen bond theory as applied to phenanthrene.
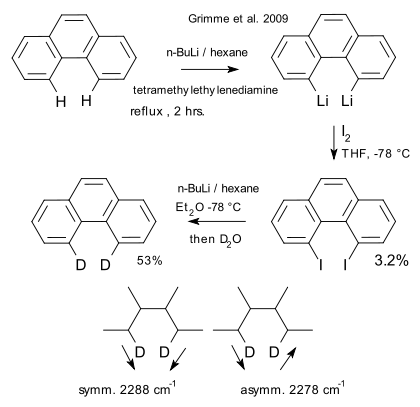
This evidence consists of infrared spectroscopy and Raman spectroscopy of phenanthrene with the two bay hydrogen atoms replaced by deuterium. Its synthesis leaves much to desire, but apparently 22 mg of it was all that was needed. The reason the researches aimed for the D isotopologue was that in the infrared spectrum two vital absorptions are well resolved: the coupled symmetric C-D stretching vibration and its asymmetric counterpart (with hydrogen many more C-H vibrations clutter spectra).
In the symmetrical mode the distance between the two deuterium atoms varies more than in the asymmetrical mode and Grimme & Erker expect a large energy difference between the two if the D-D interaction is either strongly attractive or strongly repulsive. In fact, they find a very small difference with a slightly higher energy for the symmetric mode which they argue is expected with a repulsive D-D interaction.
Grimme, S., Mück-Lichtenfeld, C., Erker, G., Kehr, G., Wang, H., Beckers, H., & Willner, H. (2009). When Do Interacting Atoms Form a Chemical Bond? Spectroscopic Measurements and Theoretical Analyses of Dideuteriophenanthrene Angewandte Chemie International Edition, 48 (14), 2592-2595 DOI: 10.1002/anie.200805751
