Palladium (II - IV) catalysis
25 February 2009 - organopalladium chemistry
The Pd(0) - Pd(II) catalytic cycle is well known in organopalladium chemistry, see for instance the Heck reaction. Less familiar is the Pd(II) - Pd(IV) cycle. An update.
A very recent report (Tsujihara et al. 2009 DOI) describes an enantioselective ring-closing reaction cyclopropanation tandem reaction of an enyne by a combination of a palladium(II) catalyst, an oxidizing reagent and a BOX ligand:
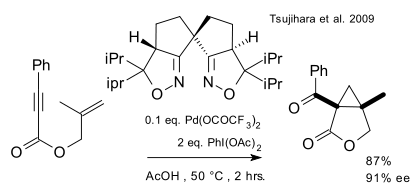
Two almost identical protocols for the racemic version of this reaction (that is, without the ligand) appeared in two articles, only separated by 6 days and both in JACS, in February 2007, one by Tong/Beller/Tse(DOI):
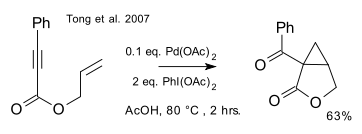
and one by Welbes/Lyons/Cychosz/Sanford (DOI):
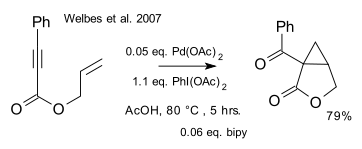
with catalytic palladium acetate and stoichiometric iodosobenzene diacetate.
The reaction mechanism both parties agreed upon is a sequence of acetoxypalladation, cyclization, oxidation of Pd(II) to a octahedral Pd(IV) intermediate by the periodinane, cyclopropanation and reductive elimination.
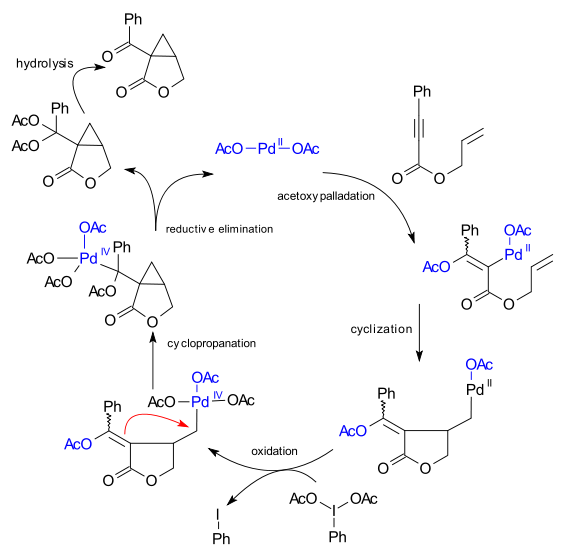 .
.
The early origins of the cyclization part of this reaction can be traced back to a Pd(II) catalysed codimerisation of an alkyne with an allyl halide (Kenada et al. 1979 DOi)
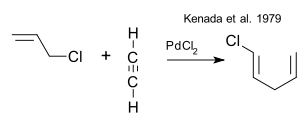
which was followed up by an intramolecular ring-closing reaction of an enyne (Lu et al. 1991 DOI)
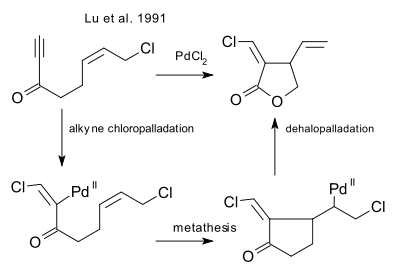
and its enantioselective version (Lu et al. 2000 DOI)
The Pd(II/IV) cycle itself has been explored by the Sanford group starting in 2004 with a C-H activation reaction of a benzoquinoline (Dick et al. 2004 DOI)

The same group went on to study oxygenation on aliphatic substrates (Desai et al. 2004 DOI), the structure of isolated Pd(IV) complexes (Dick et al. 2005 DOI), Pd(II/IV) cycles in aryl - aryl coupling reactions (Kalyani et al. 2005 DOI) and coupling reactions using oxone (Hull et al. 2006 DOI). The distinct advantage of Pd(IV) over Pd(II) in reductive elimination is the easy of forming a new C-O or C-halide bond.
The formation of an carbon-oxygen bond by reductive elimination of oxidized palladium has a history of its own. Early work is the conversion of benzene to phenol with a palladium catalyst using plain oxygen as reported by Jintoku et al. in 1990 (DOI). The methodology is ultimately based on the Shilov system (1972) which is C-H bond activation by reductive elimination not by palladium but by platinum.
Update: For Pd(III) update see here.
