Novel bismuth 3-5 catalytic cycle
24 January 2020 - Catalysis
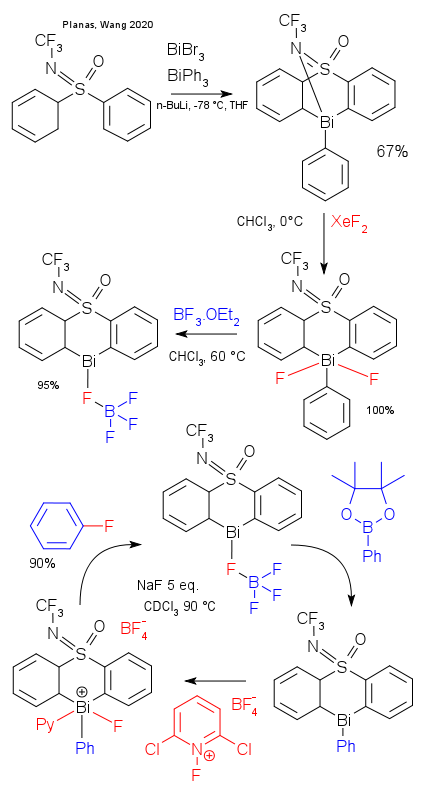 The research group of Josep Cornellà at the Max-Planck-Institut für Kohlenforschung has reported on a new use of bismuth in catalysis (Planas, Wang DOI). Cornellà has a longstanding interest in coupling reactions and it will come as no surprise the particular field of catalysis concerns fluorination of arylboronic acids. The work is extensive with a 228 page supplement. The basic idea: a Bi(III) / Bi(IV) cycle in a coupling reaction instead of the usual palladium, platinum, nickel or copper.
The research group of Josep Cornellà at the Max-Planck-Institut für Kohlenforschung has reported on a new use of bismuth in catalysis (Planas, Wang DOI). Cornellà has a longstanding interest in coupling reactions and it will come as no surprise the particular field of catalysis concerns fluorination of arylboronic acids. The work is extensive with a 228 page supplement. The basic idea: a Bi(III) / Bi(IV) cycle in a coupling reaction instead of the usual palladium, platinum, nickel or copper.
As a reminder the Suzuki reaction (typical coupling reaction) works by Ar1X (X = halide) oxidative addition to pd(0) to a Ar1-Pd(II)-X complex. In a transmetallation step the aryl group in Ar2B-(OR)3 is exchanged with the alkoxy group in Ar1-Pd(II)-OR complex to a Ar1-Pd(II)-Ar2 complex. The reductive elimination step then liberates Ar1-Ar2 and regenerates Pd(0).
The new bismuth catalyst was directly accessible from triphenylbismuth and bismuthtribromide, the authors write that the particular framework constrains the central atom to be reactive. The sulfone group is an additional ligand. In the proposed catalytic cycle the phenyl group in phenylboronic acid pinacol ester is exchanged for a tetrafluoroborate group in transmetallation. Next step is oxidative addition of 1-fluoro-2,6-dichloropyridinium tetraborate. The pyridinium ligand is then replaced by the internal bridging NCF3 ligand. In the reductive elimination step fluorobenzene is pushed out.
The catalyst loading was 10%, reported yield 90%. The reaction requires a sizeable amount of sodium fluoride, introduced as base and not additional fluoride source. Other non fluorine containing bases work but less efficient. The mechanistic role is unexplained or considered obvious.
