Novartis on Losartan
12 November 2009 - Patent literature
In the batch of latest patents (category C07 at espacenet.com) a novel method to synthesise tetrazoles by Novartis with some relevance to the production of the drug Losartan (EP2116548A1). Compared to the scientific literature where anything can be published and is published, a patent should be more relevant. A patent costs a lot of money to draw up and a scientific paper does not cost anything to have it published. As an added bonus, while the same scientific paper costs a lot of money to read (an expensive subscription to a scientific journal), the patent can be viewed for free.
The patent also has to really convince the patent office an invention is new (not known in the art) whereas with a scientific paper the peer-reviewers are fellow academic scientists and you can get away any variation that just was not tried before.
The Novartis patent in question is to the point: yes we know that tetrazoles can be made from nitriles and azides but ammonium azides are volatile with risk of explosion and organozinc azides are toxic with ecological concerns. So they have come up with dialkylboron and dialkylaluminium halides as an alternative which according to the claim has never been tried in cycloaddition with nitriles.
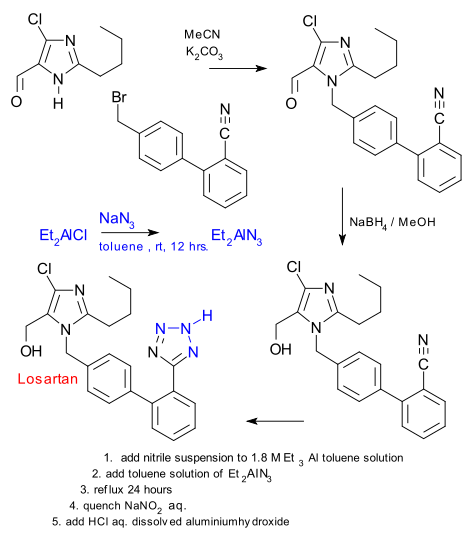
Diethyl aluminium chloride is reacted with sodium azide to (organic solvent soluble) diethylaluminium azide and this reagent reacts with a nitrile precursor to losartan. To keep the alcohol group preoccupied an excess of triethylaluminium is added first. The reaction is quenched with sodium nitrate , not reacting with the toluene solvent (another surprise according to the patent)
To be sure the patent also covers any tautomer of the tetrazole cycle as if you can isolate and sell them as unique compounds. Patent oddity.
