NNNS Chemistry blog
Prevous: Nobel Prize in Chemistry 2010
Next: Chemistry teachers discover Wikipedia
Newman-Kwart update
15 October 2010 - Mechanistically speaking
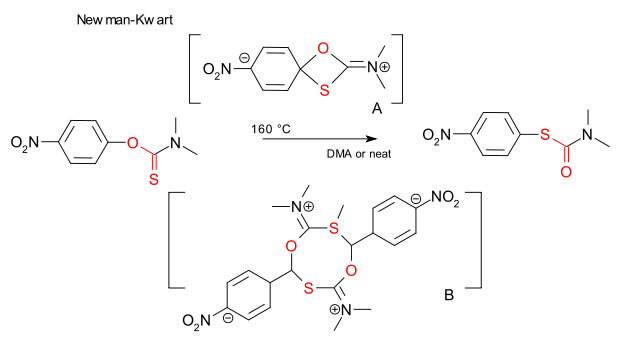 In a previous episode of this blog the Newman-Kwart rearrangement was introduced as the thermal conversion of a O-aryl N,N-dialkyl thiocarbamate to S-aryl N,N-dialkyl thiocarbamate. This reaction is known to proceed through the oxathietane intermediate A in a unimolecular reaction. In 2008 however Gilday (DOI) observed reaction rate increase with reaction concentration increase and subsequently proposed a partial bimolecular reaction though cyclic intermediate B. Recently Burns et al. stepped in to re-investigate and now reassure us it is A after all (DOI). The confusion is all about the difficulty of accurately measuring reaction rates of high temperature reactions. In contrast to the Gilday study , the Burns study did not find any variation of reaction rate with concentration. It did however notice peculiar cooling effects: a high reaction concentration involves less total reaction volume in the reaction tube leading to better exposure to the oil bath and less susceptible to cooling from the environment. Apparently the extent of fume hood sash opening already makes a difference.
In a previous episode of this blog the Newman-Kwart rearrangement was introduced as the thermal conversion of a O-aryl N,N-dialkyl thiocarbamate to S-aryl N,N-dialkyl thiocarbamate. This reaction is known to proceed through the oxathietane intermediate A in a unimolecular reaction. In 2008 however Gilday (DOI) observed reaction rate increase with reaction concentration increase and subsequently proposed a partial bimolecular reaction though cyclic intermediate B. Recently Burns et al. stepped in to re-investigate and now reassure us it is A after all (DOI). The confusion is all about the difficulty of accurately measuring reaction rates of high temperature reactions. In contrast to the Gilday study , the Burns study did not find any variation of reaction rate with concentration. It did however notice peculiar cooling effects: a high reaction concentration involves less total reaction volume in the reaction tube leading to better exposure to the oil bath and less susceptible to cooling from the environment. Apparently the extent of fume hood sash opening already makes a difference.
When the reaction mode is changed to microwave irradiation the Gilday study and the Burns study are in agreement (conversion increase with concentration) but again the devil is in the details. Burns notes that in a typical microwave experimental setup the temperature fluctuates around the set temperature. But as a higher concentrated medium (solvent is dimethylformamide) has a higher relative permittivity energy transfer is more efficient (larger Loss tangent) leading to oscillatory overheating and an apparent higher reaction rate. So who it right? Some of the Gilday team already no longer need to be convinced: Jonathan D. Moseley is co-author in both studies.
