New in Taxol
01 July 2014 - Total synthesis
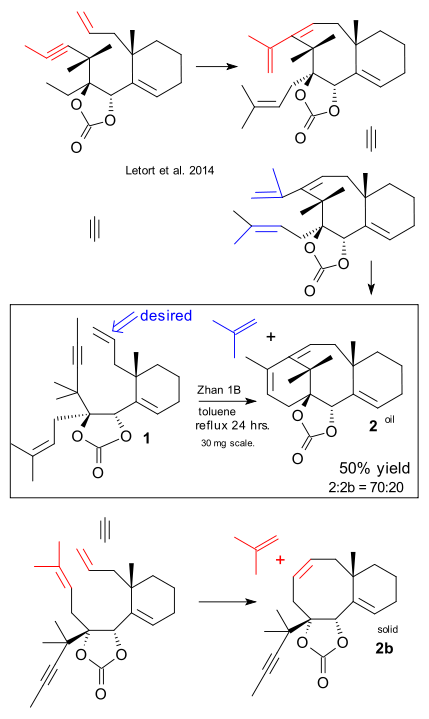 Work on taxol total synthesis continues even after 20 years. The latest exploit as reported by Letort here concerns not a new total synthesis as only the core section is synthesised or a new method as the presented cascade metathesis method has been tried before. The novelty is that this time the stereochemistry is correct.
Work on taxol total synthesis continues even after 20 years. The latest exploit as reported by Letort here concerns not a new total synthesis as only the core section is synthesised or a new method as the presented cascade metathesis method has been tried before. The novelty is that this time the stereochemistry is correct.
The precise target is molecule number 23 (of 44) of the 1994 Holton Taxol total synthesis and the method a ene yne-ene ring-closing metathesis. The chosen abbreviation, RCDEYM for this reaction has attracted some ridicule in blogland as it is not very helpful. Compound 1 is the scheme has two alkene groups and one alkyne group. A conflict of interest between the two alkene groups turned out to be troublesome. In the desired reaction path alkyne and top alkene engage in an enyne metathesis reaction followed by metathesis utimately forming compound 2. In the undesired reaction the alkyne reacts with the wrong alkene and stops there to form 2b.
In the best effort the ratio between the two compounds was 70:20. This result required using a isobutylene group for additional steric hindrance and replacing Hoveyda-Grubbs Catalyst by Zhan 1B which has an additional N,N-dimethylaminosulfonyl group.
