Memory of chirality
31 May 2008 - Stereochemistry
Enantiopure synthesis is a big research topic in organic chemistry and any racemization taking place in the course of a synthesis is usually a showstopper. For example a nucleophilic substitution at a saturated carbon atom in the SN2 regime results in inversion of stereochemistry. In the SN1 regime on the other hand the carbon center is racemized which is due to the nature of the carbocation intermediate involved. Its structure is planar and an incoming nucleophile can approach unhindered from each of the two sides. Other intermediates notable for messing up stereochemistry are free radicals and in particular enolates.
Memory of chirality (MOC) is a concept introduced in 1991 by Kawabata and Fuji where a given substrate reacts with retention of central chirality by passing through an intermediate displaying axial chirality or planar chirality (DOI DOI)
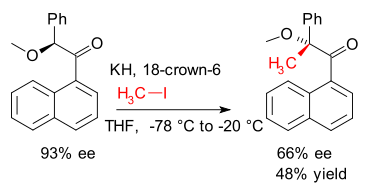
They showed how chirality was retained in an alkylation (iodomethane) of the enolate of an arylketone.
A group of French chemists have recently devised a way to synthesise new derivatives of valine also using the concept of memory of chirality ( DOI).
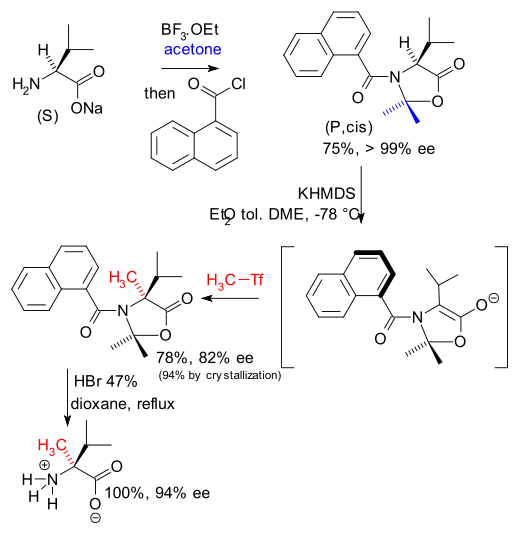
They induce temporary axial chirality in the valine enolate by converting it first to a oxazolidinone with a bulky naphthalene group. The enolate is then alkylated with MeTf and after acid hydrolysis, methylated valine is obtained with 94% enantiomeric excess.
