Kharasch-Sosnovsky Reaction
22 October 2008 - Named reactions
What: Kharasch-Sosnovsky Reaction: copper catalysed allylic oxidation using an organic peroxide.
Who: reported by M. S. Kharasch and George Sosnovsky in 1958 ((DOI) DOI). In the original publication the reactants are cyclohexene and t-butyl perbenzoate with cuprous bromide and the reaction product is cyclohex-1-en-3-yl benzoate:
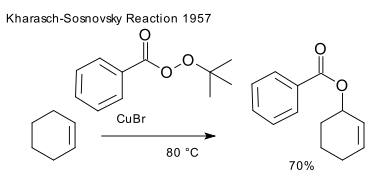
Why: many reagents react with alkenes to form dihydroxy derivatives. Free-radical based methods suffer from poor regioselectivity. One alternative method is selenium oxide based allylic oxidation.
Asymmetric: An early chiral catalysis system (1965) is based on t-butyl hydroperoxide and a chiral tartaric acid (Denney et al. DOI).Two almost identical adapted methods were reported in 1995 and based on a bisoxazoline ligand (Gokhale et al. DOI , Andrus et al. DOI). The compound (S)-cyclohexenyl benzoate thus obtained is a key intermediate in leukotriene B4 total synthesis.
Mechanism: (review: DOI). The reaction mechanism devised by Beckwith et al (DOI) contains the following steps:
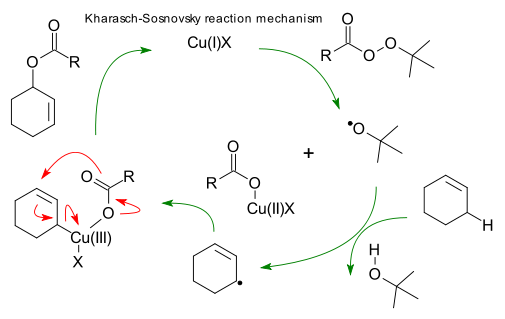
Cu(I) cleavage of the perester to a Cu(II) species and t-butyloxy radical. Radical proton exchange between allylic position cyclohexene and tBuOH. Recombination of allyl radical with Copper acetate species forming a Cu(III) intermediate and finally pericyclic reductive elimination to product and regeneration of Cu(I).
In a recent mechanistic in-silico analysis the radical intermediates are skippped in favor of a direct reaction with pre-coordinated alkene and a RCOOCu(III)OR catalyst (Mayoral et al. DOI)
Deployment: Mukaiyama Taxol total synthesis step 44 to 45 is a variation.
Organic syntheses: OS 5:70
