Industrial artemisinin
22 March 2014 - Many thanks to Bill and Melinda
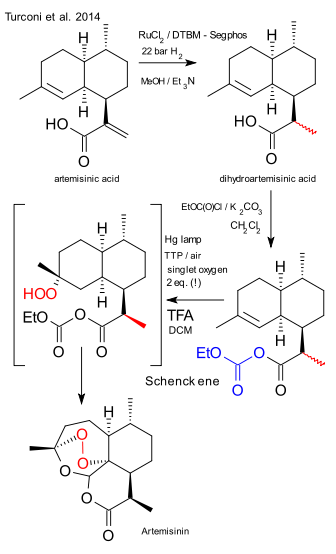 As reported back in 2012 here chemical company Sanofi and the Bill and Melinda Gates Foundation have joined forces (Sanofi the know-how and Bill the money) to increase production of the important antimalarial drug artemisinin. In a recent OPRD publication Sanofi chemists present a commercial-scale (no-loss no profit) production line with a capacity of 60 tonnes, starting from yeast-produced artemisinic acid. Here is the summary.
As reported back in 2012 here chemical company Sanofi and the Bill and Melinda Gates Foundation have joined forces (Sanofi the know-how and Bill the money) to increase production of the important antimalarial drug artemisinin. In a recent OPRD publication Sanofi chemists present a commercial-scale (no-loss no profit) production line with a capacity of 60 tonnes, starting from yeast-produced artemisinic acid. Here is the summary.
In step one from artemisinic acid to dihydroartemisinic acid (a dehydrogenation) the Wilkinson catalyst was deemed too expensive and replaced by ruthenium chloride (R)-DTBM-Segphis (a modified segphos). Scale: 600 Kg, 90% diastereoselectivity. The compound was next activated with ethylchloroformate and potassium carbonate in dichloromethane to the anhydride. The photochemical step consisted of adding tetraphenylporphyrin as a sensitizer and trifluoroacetic acid in dichloromethane. The subsequent Schenck ene reaction / Hock rearrangement requires two equivalents of singlet oxygen. Where the prior art yielded 41% of product, this photochemical solution pushes out 55%. Side note: the article does not really explain why the acid was activated, the Seeberger procedure does not include this step. Remaining challenge: product isolation was accomplished by simultaneous DCM distillation - solvent replacement with n-heptane and crystallisation. Pretty amazing when considering this is still industrial production at the hundreds of kilogram scale and the final product is a labile peroxide!
Please do admire the photochemical reactor pic embedded in the article. At the Sanofi plant in Garessio (Italy) it is Christmas every day of the year.
