Grignards on lithium
15 October 2008 - news
The standard routine for synthesising Grignard reagents is adding magnesium metal to a suitable halocarbon. Another method (discovered by Prevost in 1931) is by metal-halogen exchange of a halocarbon with another (simple) Grignard reagent where the X on RX trades places with the MgX on RMgX. In 2004 Knochel found that this particular reaction is accelerated when the Grignard is complexed with simple lithium chloride (DOI). The basics.
The (now commercial Link) THF solution of isopropylmagnesium chloride with lithium chloride (CAS 807329-97-1) is sometimes called a turbo-Grignard.
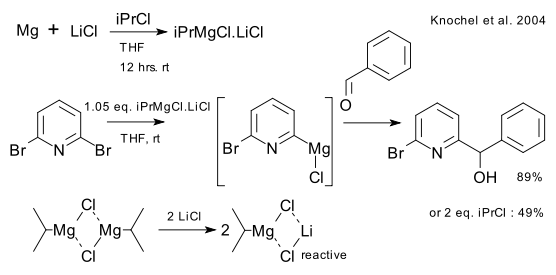
This reagent is prepared by simply mixing magnesium , isopropyl chloride and lithium chloride in THF. Its enhanced reactivity is attributed to breaking up RMgX polymeric aggregates, improving solubility and R3Mg-Li+ ate complex formation. Adding a crown ether speeds up certain reactions even further by isolating the lithium ion and increasing the negative charge (DOI).
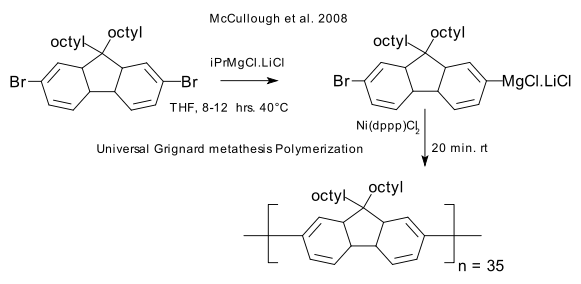
The synthetic utility of Grignards on lithium have been demonstrated in manipulations on aromatic carboxylic acids (DOI). Buchwald examined its use in a Kumada coupling (DOI) and a 2008 patent (Carnegie Mellon University, Link) describes so-called Universal Grignard metathesis Polymerization (GRIM) for application in conjugated polymers (solubility is always key in polymerization) for instance in a poly(fluorene):
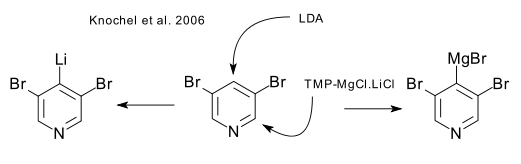
In 2006 Knochel synthesized a magnesium superbase variation on lithium tetramethylpiperidide which owing to the presence of LiCl is very THF soluble (DOI). Bases of this type are sometimes called Hauser amides (DOI DOI) after C. Hauser who in 1949 used amides of this type in certain ester condensations.
Most recently in 2008 Garcia-Alvarez et al. determined by x-ray crystallography the molecular structure of this base, which is found to have a nonplanar LiClMgCl ring with the TMP part firmly attached to Mg (and not Li) and with no less than three solvatating THF molecules (DOI).
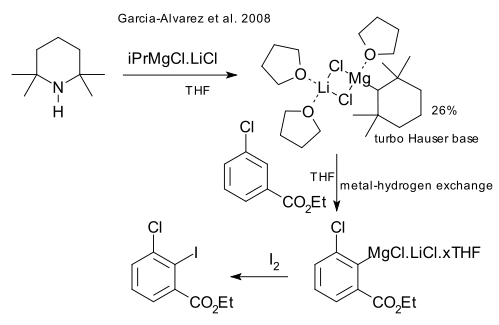
From it is confirmed that it is a molecular halide and not a salt.
