Easiest alkene hydration ever
5 January 2009 - News
The laboratory of Guy Bertrand has synthesised a new variety of fulvalenes, the compounds characterized as cyclic polyenes yet not aromatic and exhibiting cross-conjugation (Kinjo et al. DOI). Although the new compound is stable at ambient temperature and even melts without complaining at 130°C owing in part to a hydrocarbon kinetic shield, oxygen is toxic and the central alkene group is found to react spontaneous with water: the easiest alkene hydration ever.
Remarkably the compound (as the cis and trans isomer) is synthesized from tetrachlorocyclopropene in good yield in four reaction steps (each as old as chemistry) but without any serious intermediate workup/purification steps. It can almost be mass-produced. Step one is a double Friedel-Crafts reaction first with bulky triisopropyl benzene (Tip) and next with less-bulky mesitylene (mes) forming 3. Phosgene reinstates the chlorine groups and Wurtz type reaction with magnesium clenches the deal.
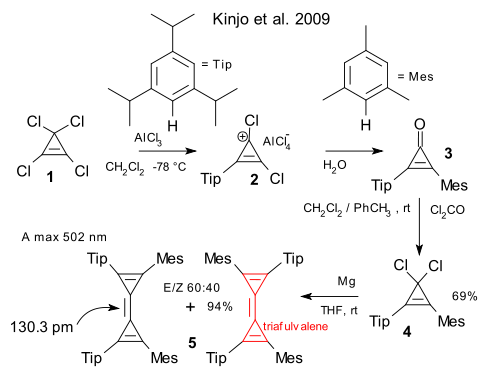
The triafulvalene skeleton is almost planar, the central alkene is found to be extremely short and in UV/VIS the absorption present in ethylene at 171 nm is now redshifted to 502 nm.
