Cyclobutadiene X-ray structure debate continues
22 March 2013 - Crystallography
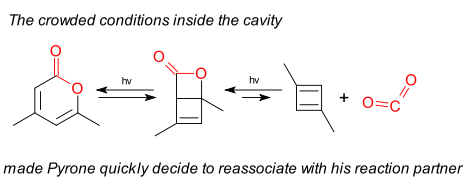 Opposition to the cyclobutadiene X-ray structure claimed by Yves-Marie Legrand in 2010 (DOI) continues. According to Legrand 1,3-dimethylcyclobutadiene formed by photolysis of a pyrone is stable when confined to a calixarene cavity and when analyzed at 175K displays two geometries, one square-planar and one rectangular-bent. Carbon dioxide is also present in the cavity as a reaction product but is hydrogen-bonded at the most. Rzepa in 2010 was quick to blog his doubts: cyclobutadiene when formed quickly recombines with CO2 to a Dewar benzene like compound (earlier blog here). The same opposition was aired by Scheschkewitz (DOI) and Alabugin et al. also in 2010 (DOI). Both opponents noted the CO2 molecule in the crystallographic data is unusually bent (111°C) something Legrand and the reviewers had apparently missed.
Opposition to the cyclobutadiene X-ray structure claimed by Yves-Marie Legrand in 2010 (DOI) continues. According to Legrand 1,3-dimethylcyclobutadiene formed by photolysis of a pyrone is stable when confined to a calixarene cavity and when analyzed at 175K displays two geometries, one square-planar and one rectangular-bent. Carbon dioxide is also present in the cavity as a reaction product but is hydrogen-bonded at the most. Rzepa in 2010 was quick to blog his doubts: cyclobutadiene when formed quickly recombines with CO2 to a Dewar benzene like compound (earlier blog here). The same opposition was aired by Scheschkewitz (DOI) and Alabugin et al. also in 2010 (DOI). Both opponents noted the CO2 molecule in the crystallographic data is unusually bent (111°C) something Legrand and the reviewers had apparently missed.
In their 2010 rebuttal Legrand et al. (DOI) argue that in many studies dealing with very unstable molecules the data tend to get messy and that in that respect our data are no worse than these studies, if not better. They also note that bent carbon dioxide has been observed in other systems.
In 2011 the Legrand team took a step further: they presented evidence (NMR, MS) that 1,3-dimethylcyclobutadiene (still carcerand confined) is stable in water at room temperature for several weeks and even up to 50 °C
This year Rzepa (DOI) presented more computational evidence against the Legrand model and also this year Shatruk and again Alabugin reinvestigated the original crystallographic data (DOI). Again no cyclobutadiene.
