Citalopram battle
28 January 2009 - News
Researchers from Dr. Reddy's Laboratories and Lundbeck have been exchanging letters in Organic Process Research & Development (OPRD) on the nicer details of citalopram production. This (racemic) antidepressant drug was invented 1989 by Lundbeck and after the patent expired in 2003, Dr. Reddy's went to the market with their own generic version while Lundbeck came up with the more potent (S)- enantiomer now called escitalopram. In these exchanges the Lundbeck scientists report on inconsistencies they found in a 2007 Dr. Reddy's article (Elati et al. DOI) regarding the synthesis of chiral citalopram. There is no clear winner yet although the editorial board and referees of OPRD should take some blame where they do not. Here is what happened.
Two elements in the 2007 article by Dr. Reddy's team that cause all the commotion are the chiral resolution by diastereomeric recrystallization of citalopram using (-)-di-p-toluoyltartaric acid (DPTTA) and the alkylation of an citalopram intermediate.
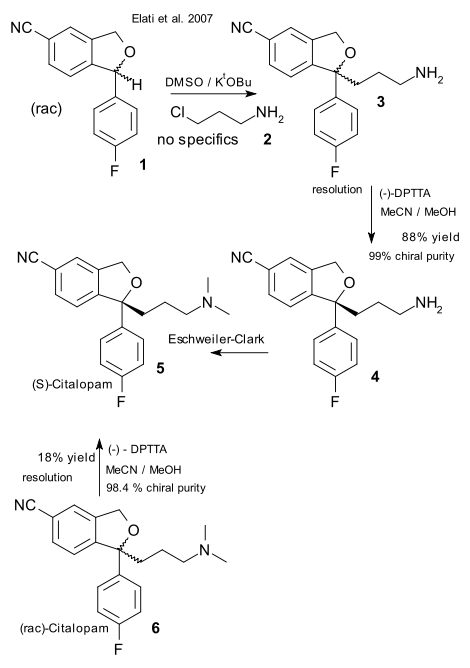
The Dr. reddy's team state at the onset that this chiral resolution method is not feasible on a plant-scale but nevertheless report a 18% yield with 98% of what is called chiral purity in the experimental section although the article itself does not discuss this result. The Lundbeck team asserts (DOI) that this method cannot work and spent some effort to repeat the results but without luck: the solubility of the racemate is simply too poor.
In their reply (DOI) the Dr. Reddy's team concede that their report did contain errors on three accounts: firstly the desired (S)-enantiomer is isolated not from the crystalline phase (the textbook scenario) but from the mother liquor, secondly not (-)-DPTTA is used but the other enantiomer (+)-DTTTA and lastly the filtrate is neutralized and the free base is extracted with toluene. On the other hand the team did report a corrected procedure and again did obtain enantiopure (S)-citalopram from racemic citalopram (11% percent yield , 96.4% chiral purity repeating the procedure twice).
In their reply to a reply (aptly called Response to the Comments by Elati et al. in Response to Our Article Examining One of Their Previous Articles) the Lundbeck team is still unconvinced (DOI). Again they repeat the resolution and this time obtain 76% enantiomeric excess with 7% yield in the second crystallisation. A third one (described by the Dr. Reddy's team) utterly fails.
The other topic of contention, the alkylation of the benzofuran intermediate by chloropropylamine is mentioned in the 2007 article but surprisingly only described in a patent (WO Patent 047274). The Lundbeck team questions the preparation of the amine as a free base which is known to rapidly polymerize to a polyamine and also question the use of a acetone / potassium tert-butoxide mixture which not only is known to react violently but is also known to yield only the aldol reaction product. In their reply The Dr. Reddy's team present their optimised procedure (6 Kg scale!) which involves adding the free base quickly to a benzofuran / DMSO (not acetone) / tBuOK mixture.
In an editoral(DOI) the OPRD concludes that the process is complete and that they trust our readers will gain a better understanding of a difficult situation and that we have illustrated a mechanism for handling similar disagreements that we may face in the future.. This blog is not so sure.
Why are the OPRD and its referees to blame: the reported chiral resolution of citalopram in the 2007 article should have been left out as it was unrelated to its main topic: the resolution of an intermediate. They further allowed the discussion to get out of control by having the Lundbeck team engage in lengthy discussion on patent literature. In addition they started a new round of criticism in their second reply, this time concerning the resolution of the primary amine intermediate. The referees also allowed the fuzzy unit of chiral purity to persist.
The discussion will probably resume in patent court.
Robert James Dancer, Heidi Lopez De Diego (2009). Response to the Comments by Elati et al. in Response to Our Article Examining One of Their Previous Articles Organic Process Research & Development, 13 (1), 38-43 DOI: 10.1021/op800252w
