Chemists embrace nuclear energy
10 September 2010 - Well, at least two of them
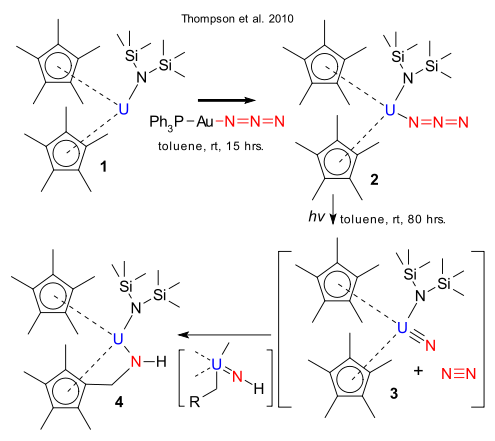 Uranium nitride (UN)x is an alternative nuclear fuel that outsmarts competing uranium trioxide because of higher melting point and better thermal conductivity. And where ever two elements combine (uranium and nitrogen), chemists are just around the corner. Thompson at al. of the Los Alamos National Laboratory (where else) looked at ways to generate a terminal uranium nitride (RUN, transient or not) and its fate (DOI). Compound 1 is a compound of uranium with two pentamethylcyclopentadienyl ligands and a diisopropylamide ligand. It can be oxidized by the gold azide Ph3PAu(N3) to uranium(IV) compound 2 which at photolysis forms amide 4 and according to Thompson through C-H activation of one of the Cp* methyl groups via transient uranium nitride 3. According to DFT computing the favored reaction mechanism is proton abstraction by the nucleophilic nitride unit and formation of a temporary C-U bond followed by a 1,2 methylene group migration over the U-N bond. The similarity of this reaction with that of Fe=O with C-H bonds in Cytochrome P450 is noted.
Uranium nitride (UN)x is an alternative nuclear fuel that outsmarts competing uranium trioxide because of higher melting point and better thermal conductivity. And where ever two elements combine (uranium and nitrogen), chemists are just around the corner. Thompson at al. of the Los Alamos National Laboratory (where else) looked at ways to generate a terminal uranium nitride (RUN, transient or not) and its fate (DOI). Compound 1 is a compound of uranium with two pentamethylcyclopentadienyl ligands and a diisopropylamide ligand. It can be oxidized by the gold azide Ph3PAu(N3) to uranium(IV) compound 2 which at photolysis forms amide 4 and according to Thompson through C-H activation of one of the Cp* methyl groups via transient uranium nitride 3. According to DFT computing the favored reaction mechanism is proton abstraction by the nucleophilic nitride unit and formation of a temporary C-U bond followed by a 1,2 methylene group migration over the U-N bond. The similarity of this reaction with that of Fe=O with C-H bonds in Cytochrome P450 is noted.
As an aside the Thompson article in Nature Chemistry happily endorses nuclear energy as a Weapon Against Global Warming. In an accompanying editorial commentary Paula L. diaconescu is equally enthusiastic, that makes two!
