Chemical hydrogen storage latest
16 June 2009 - recent literature
Just in case you have been hiding under a rock for the past decade: hydrogen storage, an integral part of the hydrogen economy is hot!. One particular branch of hydrogen storage that attracts the attention of chemists (and research funds) is chemical storage and it seems no element in the periodic table is left behind when it comes to ingenious solutions. Here is a pick just from the recent literature.
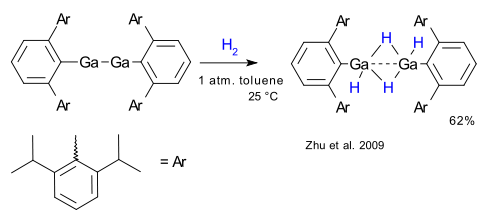
First up is gallium. All elements in the boron group are able to react with small molecules such as hydrogen and ammonia and gallium is no exception as in the aryl Ga(I) reaction depicted below (Zhu et al. 2009 DOI)
Tetrahydroborates are reducing agents but also potential hydrogen donors with lithium borohydride containing up to 20% hydrogen by weight. This compound reversibly decomposes to LiH and B. Problem is that the reaction is endothermic and temperatures in excess of 400 °C are required to release the hydrogen. So destabilizers are added for example in a system recently explored containing LiBH4, CaH2 and additive V2O5 (Ubikunle et al. DOI) . The authors are quick to remark that trying out all these chemical in different formulations can "be quite time consuming" but at least the reaction CaH2 + 6 LiBH4 -> 6LiH + CaB6 + 10H2 was the result of prior computer modelling.
A new solid-state synthesis for LiBH4 (wet-chemistry procedures exists) is also reported recently using lithium hydride and diborane at 120 °C (Friedrichs et al. DOI). This blog however struggles to understand the need to source the diborane from a reaction of zinc chloride with ahum.. said lithium borohydride, according to 2LiBH4 + ZnCl2 -> Zn(BH4)2 + 2LiH (by milling) followed by heating Zn(BH4)2 to gaseous Zn + B2H6 + H2. Going round in circles.
lanthanum, praseodymium, neodymium, magnesium, nickel and aluminum join forces to bring you the alloy La0.55Pr0.10Nd0.12Mg0.23Ni3.4Al0.1 with again, surprising hydrogen storage capabilities (Huizhong et al. DOI). This kind of research must be tedious to the extreme: the number of formulations with just these 6 elements is almost infinite and on top of that the alloys formed are multiphasic. Computer modelling is useless in predicting any atomic packing - surely one of those unsolved problems in chemistry - let alone predicting additional hydrogen storage capacity.
Carbonic materials can also be adapted for hydrogen storage. In a recent report, nitrogen enriched graphite is synthesized by mixing cyanuric chloride and melamine with pyridine in DMF at room temperature followed by curing at 120 °C (Yang et al. 2009 DOI). The experimental hydrogen capacity is 0.34% at 100 bar and 298 K and not exactly meeting the standard for practical implementation of 6.5% by weight.
The hydrogen storage capacity of nanotubes can be improved by creating defects for example by decorating them with metal nanoparticles. The process responsible is called hydrogen spillover :the metal splits diatomic hydrogen into monoatomic hydrogen which then enters the nanotube. A recent report reduces silver nitrate with hydrizine in a nanotube suspension (Rather et al. DOI) resulting in metal coated nanatubes with 0.86% H2 capacity (still modest).
Metal-organic frameworks are porous composite materials ideally suited for hydrogen storage and a lot of research is invested into this particular field. Covalent organic frameworks (COF's) are less common and the trick is to find a rigid organic molecule that forms channels in their crystal structure just as in a zeolite. In one report, the Cambridge Structural Database was mined (or the CSD "desert was sieved" according to the authors) to produce a biphenyl compound that did fit the bill and the hydrogen capacity was found to be 0.80% at 10 bar (Msayib et al. DOI)
