Chemical graphiti
21 August 2010 - Nanotechnology
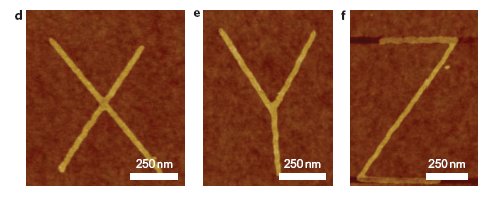 Chemists occasionally fool around as graffiti artists, see an earlier episode here. Xinran Wang and Hongjie Dai (Stanford university) have been making some graffiti of their own using graphene or rather graphene nanoribbons (DOI). With regular electron beam lithography these ribbons are just too wide (20-50 nm) and outside the range for good electronic properties (sufficient band gap). Wang & Dai exposed the ribbons to a low-pressure mixture of oxygen , ammonia and argon at temperatures of up to 800°C and observed an etching process with carbon being eaten away from the edges with a typical speed of 1 nanometer per minute. In the mechanistic picture oxygen dissociates and reacts with surface carbon atoms preferably the more reactive edge carbon ones. In step two carbon monoxide or carbon dioxide dissociates removing one carbon. The addition of ammonia is designed to slow down the edging process to manageable speeds. It does so by reducing away surface bound oxygen interrupting the process.
Chemists occasionally fool around as graffiti artists, see an earlier episode here. Xinran Wang and Hongjie Dai (Stanford university) have been making some graffiti of their own using graphene or rather graphene nanoribbons (DOI). With regular electron beam lithography these ribbons are just too wide (20-50 nm) and outside the range for good electronic properties (sufficient band gap). Wang & Dai exposed the ribbons to a low-pressure mixture of oxygen , ammonia and argon at temperatures of up to 800°C and observed an etching process with carbon being eaten away from the edges with a typical speed of 1 nanometer per minute. In the mechanistic picture oxygen dissociates and reacts with surface carbon atoms preferably the more reactive edge carbon ones. In step two carbon monoxide or carbon dioxide dissociates removing one carbon. The addition of ammonia is designed to slow down the edging process to manageable speeds. It does so by reducing away surface bound oxygen interrupting the process.
