Carbon dioxide fixation latest
25 January 2010 - Organic chemistry
In the continuing coverage of chemical carbon dioxide fixation coverage (see earlier episode here) three recent contributions from Spain, The Netherlands and Japan. Sanz et al. (DOI) have been exploring catalytic reduction of carbon dioxide with hydrogen (60 atm) and a base (KOH/water) to the formate ion. Key is adding a NHC ligand as strong electron donor to iridium catalyst Cp*Cl2Ir. Using isopropanol instead of hydrogen in transfer hydrogenation also works with an turnover number of 150. As an added bonus the formic acid/formate system is also in the picture as an hydrogen storage vehicle. This probably means getting a research grant is twice as easy.
Angamuthu et al. (DOI) have not formate but oxalate on their mind as a form of fixated carbon dioxide. Their catalyst is based on Copper(II) acetylacetonate with copper(II) reduced to copper(I) with addition of an thiol (forms a disulfide) containing ligand. Unexpectedly this compound was found to be oxidized by carbon dioxide and not by oxygen. A system was then devised with spent copper(II) reduced back electrochemically which process turned out to be more favorably than direct CO2 reduction
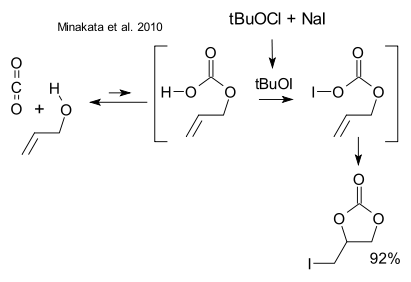
Minakata et al. (DOI) too claim to have found a new way to capture carbon dioxide before it can destroy our climate. They exploit an old reaction reaction in a novel way: carbon dioxide is known to react with simple alcohols (for example allyl alcohol ) to form an alkyl carbonic acid but the chemical equilibrium is unfavorable. Solution: the unstable carbonic acid RO(CO)OH is iodinated with sodium hypoiodite to RO(CO)OI which then reacts in a kind of halolactonization to an organic carbonate.
:
But where formate and oxalate salts have some utility in their own right it is difficult to see genuine bulk applications for the particular cyclic carbonate formed. In this respect the Japanese seem to have lost the plot.
