Biggest catalyst ever
24 March 2012 - Catalysis
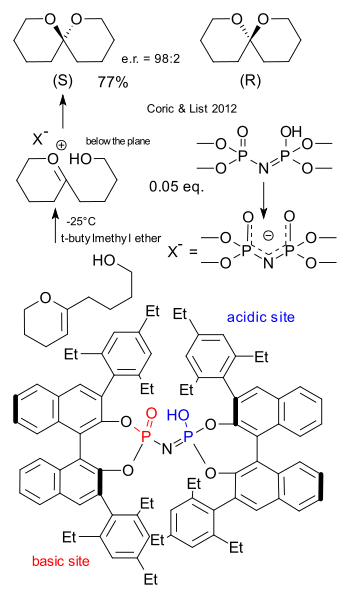 Ilija Coric and Benjamin List must have made the biggest chiral catalyst ever. The thing did not even fit on one of the pages of the letter to nature (DOI)and even in the supplemental information it looks highly distorted. Only enzymes are bigger but that is exactly what Corin and List had in mind: creating a synthetic catalyst that works like an emzyme (biomimetics) , rigid, completely encapsulating the substrate with an internal cavity that is as small as possible for maximum chiral induction. The main building block is the well-known chiral binol. The core is a imidodiphosphoric acid that has a basic site as well as a acidic site. When this Brønsted acid catalyst donates a proton the residue (X-) is a C2 symmetric chiral anion (concept introduced by Toste in 2007). The substrate is a vinyl ether and after accepting a proton the intermediate is a oxocarbenium ion. The delta-hydroxy group attached to it can add from the top of the plane or from below the plane depending on the chirality of the catalyst. The resulting spiro compound in this enantioselective synthesis is unremarkable except that it is a sex pheromone for the olive fruit fly. The (R) isomer attracts only the males and the (S) isomer only the females. We can only hope the List laboratory is a safe distance away from any olive orchard.
Ilija Coric and Benjamin List must have made the biggest chiral catalyst ever. The thing did not even fit on one of the pages of the letter to nature (DOI)and even in the supplemental information it looks highly distorted. Only enzymes are bigger but that is exactly what Corin and List had in mind: creating a synthetic catalyst that works like an emzyme (biomimetics) , rigid, completely encapsulating the substrate with an internal cavity that is as small as possible for maximum chiral induction. The main building block is the well-known chiral binol. The core is a imidodiphosphoric acid that has a basic site as well as a acidic site. When this Brønsted acid catalyst donates a proton the residue (X-) is a C2 symmetric chiral anion (concept introduced by Toste in 2007). The substrate is a vinyl ether and after accepting a proton the intermediate is a oxocarbenium ion. The delta-hydroxy group attached to it can add from the top of the plane or from below the plane depending on the chirality of the catalyst. The resulting spiro compound in this enantioselective synthesis is unremarkable except that it is a sex pheromone for the olive fruit fly. The (R) isomer attracts only the males and the (S) isomer only the females. We can only hope the List laboratory is a safe distance away from any olive orchard.
