Bent phenyls
3 July 2008 - Organic chemistry
Bending benzene rings is nothing new. The record bend in basket shaped cyclophanes stands at 21°. The molecule depicted below (Strohmann et al. 2008 DOI) is not a cyclophane but nevertheless odd.
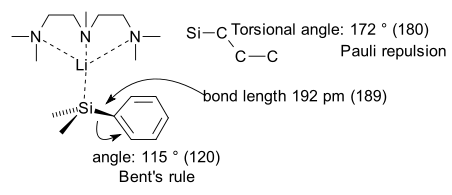
It is the silicon analogue of a benzyl anion with two additional methyl groups on silicon and a lithium counterion which is coordinated to PMDTA.
Whereas a benzyllithium is planar with the lone pair in a p-orbital, silyllithiums have increased s-character with corresponding pyramidalization.
X-ray crystallography for this compound reveals a longer than usual Si-C bond length, a smaller than usual Si-C-C angle attributed to Bent's rule and surprisingly a Silicon dihedral angle of 172 ° which makes the benzene ring bent. As an interesting generalization this bending is found to take place with all higher elements in the carbon group and the nitrogen group.
The researchers explain this bending by Pauli repulsion between thefrontier orbital on silicon and the one on the arene. This repulsion is loosely related to the Pauli exclusion principle in which electrons in two filled orbitals tend to repulse each other at short range because they cannot occupy the same state. The frontier orbital of silicon can avoid alignment and overlap by this bending mode.
Only problem with this explanation is that the phenyl group flips towards the silicon lone pair. Simply flipping away would also put an end to steric concerns
