(-)-cyanthiwigin F total synthesis
6 July 2008 - Organic chemistry
The (-)-cyanthiwigin F total synthesis (Enquist, Stoltz 2008 DOI) featuring a double catalytic enantioselective transformation made headlines this month. What is this process all about and what makes it so special?
The molecular target in any case did not warrant urgent synthetic synthesis: it is one of many known cyanthiwigins and part of an even larger class of cyathins. Millions of compounds display some kind of biological property that can potentially be used in medicine. The molecule is a fused tricyclic system with 4 stereocenters, two of which are quaternary which makes synthesis tricky.
Here is how it was done. Step one is a Claisen-Dieckman condensation (first inter- then intracondensation) of the di-allyl ester of succinic acid. The acidic protons in the 1,3-diketone groups can be replaced by a methyl group with iodomethane and a base. These reactions are not very stereospecific and intermediate 3 is a diastereomeric mixture of racemic (R,R) and (S,S) and meso (R,S).
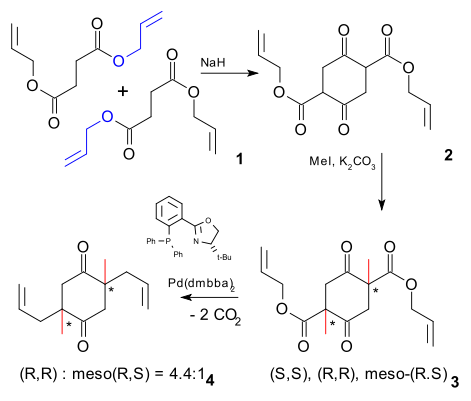
In a traditional approach this reaction is a failure because the desired enantiomer (R,R) is contaminated by an equal part of (S,S) (the meso contamination can be dealt with). In this approach the researchers decided to forge ahead with the next step, a decarboxylative allylation on the mixture itself.
Surprisingly the reaction product 4 is a mixture of desired (R,R) and meso (R,S). What happens is that twice in this reaction a carboxylic ester group is replaced by an enolate ion, destroying a stereocenter (process called stereoablation). The outcome of the reaction (16 possible pathways) is then governed by the stereochemistry of the BOX ligand and the stereochemistry of the remaining stereocenter.
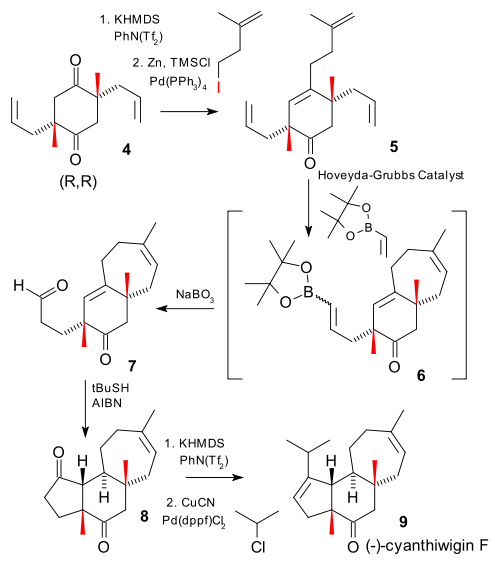
In the remainder of the reaction an olefinic side group is attached via a Negishi coupling to tetraene 5, the second generation Hoveyda-Grubbs Catalyst closes a 7-membered ring in 6 and at the same time functionalises the remaining alkene with a vinyl boronic ester. This product is then oxidized to aldehyde 7 with sodium perborate. The final steps are a radical addition (of recent 2005 invention DOI) joining the acyl group with the internal alkene, and a palladium catalyzed organocopper addition that almost fails due to excessive reduction.
So are double enantioselective reactions are viable strategy in organic synthesis? It seems the stereochemical outcome of this particular reaction was one governed by chance rather than by design, so no need to get al excited.
Another aspect this reaction is new: it does away with protective groups. The authors note that PG-free reactions are still rare in total synthesis and interestingly cite Robinson's classic tropinone synthesis of 1917 and Barans Hapaindole work of 2007 (fast becoming a classic although you might think otherwise if you believe the commentators at Wikipedia's protective group talk page).
The message clearly is not to dispair when faced with ugly reaction mixtures or destroyed functional groups but simply go ahead and repair the damage along the way (also see previous post on Prostratin total synthesis).
