Two views on anti-Markovnikov Wacker oxidation
01 July 2012 - Catalysis
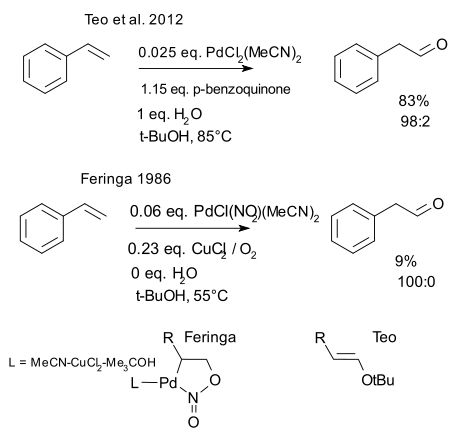 Chemistry can be a slow-paced science when it comes to sorting out specific problems. Take for example the anti-Markovnikov Wacker-Tsuji oxidation of styrene. Peilli Teo of the grubbs lab has just published (DOI) a novel variation to a procedure first established in 1986 by Ben Feringa (DOI).
Chemistry can be a slow-paced science when it comes to sorting out specific problems. Take for example the anti-Markovnikov Wacker-Tsuji oxidation of styrene. Peilli Teo of the grubbs lab has just published (DOI) a novel variation to a procedure first established in 1986 by Ben Feringa (DOI).
In a regular Wacker oxidation an alkene is oxidized to a ketone with a palladium(II) catalyst in presence of water. Pd is continuously regenerated by copper chloride which is in itself regenerated by oxygen so the reaction is potentially cheap to run as none of the metals is actually consumed. The addition however is Markovnikov because somewhere a carbocation must be accommodated and hence the ketone is formed. So how to get to the aldehyde instead?
Feringa in 1986 only had to replace one chlorine ligand by a nitro ligand in the catalyst and replace solvent DMF by tert-butanol to make it all happen. As often happens in chemical research this finding was entirely "unexpectedly". We have to admire Feringa's sense of creative accounting. The yield is appalling (just 9%) so the main table only has the yields listed in mmoles (you have to do the math yourself). One claim mentioned in the text itself reports a fantastic 280% yield but that one is measured against palladium of course.
So how has the Grubbs team improved on this procedure? Copper and oxygen have been replaced by stoichiometric p-benzoquinone, the original Pd catalyst is restored and very smartly: they added water. The isolated yield jumps to 83% and the team notes that although adding water is not strictly needed (tBuOH is wet enough) without it, the yield drops again to 38%. Feringa reported adding water did nothing.
Mechanistically there is not a lot of agreement either. Feringa has a prominent role for the nitro ligand to play. The PdNO pentacycle falls apart in the beta-elimination. In the Grubbs model the first intermediate is the tert-buyl vinyl ether which is then hydrolysed. Considering the steric bulk involved only the Grubbs model makes sense.
