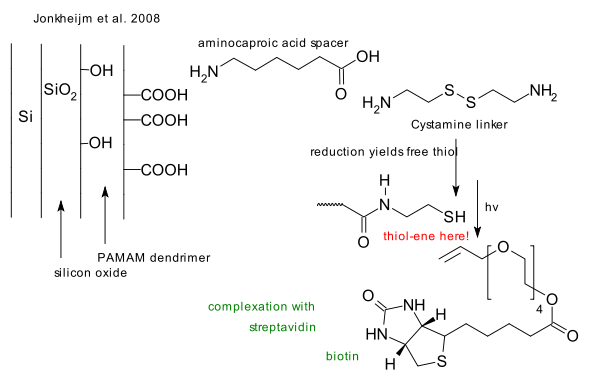
The thiol-ene reaction
3 November 2008 synthetic methods
What: The thiol-ene reaction is an organic reaction between a thiol and an alkene forming a thioether.
Discovered: Theodor Posner 1905 ( DOI)
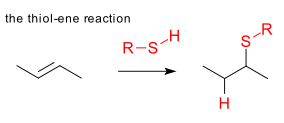
Mechanism: In absence of initiators the reaction is an electrophilic addition obeying Markovnikov's rule very much like alcohols react to alkenes. In presence of a radical initiator such as AIBN or in a photochemical reaction the reaction type is anti-markovnikov free radical addition (Jerry March 1986). Reactions of alkenes with hydrogen sulfide also lead to thioethers through the intermediate thiol.
Use: Used extensively in polymer chemistry as crosslinking reagent and most recently in click chemistry (DOI). Key advantages: metal-free reactions and 100% atom economy.
Organic syntheses: III:458 & IV:669
Scope: Just a few examples from recent literature. Thiol-ene polymerization based on cyanuric acid and pentaerythritol was used in photolithography in a nanoelectronics application (Khire et al. DOI) citing better resistance to oxidation, lower schrinkage and more complete chemical conversion than existing network systems based on for instance acrylate monomers:
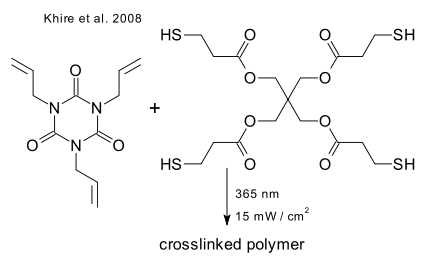
In another recent example (Jonkheijm et al. DOI) the outer layer of a sheet of silicon is converted to silicon oxide ([[chemical vapor deposition), attached is a layer of PAMAM dendrimer, to which is attached a amino caprioc acid spacer, to which is attached a cystamine linker, to which is attached (after cleavage of the disulfide bond) via thiol-ene coupling an alkene functionalized biotin molecule which can bind to streptavidin: