The nitrogen splicer
15 May 2021 - Precision synthesis
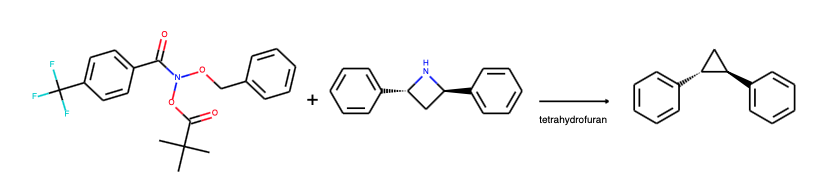 The laboratory of Mark Levin is reporting a new type of organic reaction in which the nitrogen unit of a secondary amine can be removed with a simple N-benzyloxy-N-alkoxy-benzamide (Kennedy et al. DOI). The Levin group has 'precision synthesis' and 'skeletal editing' listed as core research interests and hence the new research is not a coincidence. Now, if skeletal insertion reactions are rare, the Beckmann rearrangement and the Baeyer-Villiger oxidation come to mind, skeletal deletions are the unicorns of synthetic chemistry. The Favorskii rearrangement and the Wolff rearrangement are two of them. Of course biochemists make it all look easy with their RNA splicing and so forth but that has more to do with luck than hard work as we all very well know. The phrase skeletal editing seems to have debuted in this article, the only thing coming close is "late stage functionalization" (review here). It must be nice to be able to take any existing drug, be able to insert or remove heteroatoms at will and create a new drug.
The laboratory of Mark Levin is reporting a new type of organic reaction in which the nitrogen unit of a secondary amine can be removed with a simple N-benzyloxy-N-alkoxy-benzamide (Kennedy et al. DOI). The Levin group has 'precision synthesis' and 'skeletal editing' listed as core research interests and hence the new research is not a coincidence. Now, if skeletal insertion reactions are rare, the Beckmann rearrangement and the Baeyer-Villiger oxidation come to mind, skeletal deletions are the unicorns of synthetic chemistry. The Favorskii rearrangement and the Wolff rearrangement are two of them. Of course biochemists make it all look easy with their RNA splicing and so forth but that has more to do with luck than hard work as we all very well know. The phrase skeletal editing seems to have debuted in this article, the only thing coming close is "late stage functionalization" (review here). It must be nice to be able to take any existing drug, be able to insert or remove heteroatoms at will and create a new drug.
As to the what, the innovation boils down to a successful reaction of 'deletion reagent' N-(benzyloxy)-N-(pivaloyloxy)-4-(trifluoromethyl)benzamide with substrate bis(4-bromobenzyl)amine to product 1,2-di(4-bromophenyl)ethane. Same substrate minus the amino group. In the same way an azetidine can be converted to a ring-contracted cyclopropane. The reagent has an 1.5 equivalent excess, the reaction conditions are THF, 45 degrees Celsius and 14 to 16 hours reaction time.
As to the how, Levin explains the active intermediate must be a so-called isodiazene, which is if I look at it, a diazonium compound reunited with it's organic counterpart as in a neutral R-N(=N)-R'. This compound type is known for its ability to expel dinitrogen with recombination of the two R and R' radical groups. As to the when, it is explained that inspiration was drawn from a 1998 article on so-called anomeric amides. The anomeric effect as working on an amide with two oxygen neighbors will cause the geometry of the amine group to change in a way that it becomes chemically activated.
That brings us to the buts. The deletion reagent is also an oxidizer which rules out for example thiols as substrates. Competing radical fragmentation reactions can occur instead of the desired radical recombination. The reaction is of course not very atom efficient but the splicer must be just the first-generation reagent. There must be a catalytic cycle somewhere.
