NNNS Chemistry blog
Prevous: DIY solvated electrons
Next: Dutch chemistry departments: we are fantastic!
The chiral Grignard
27 April 2011 - Orgo
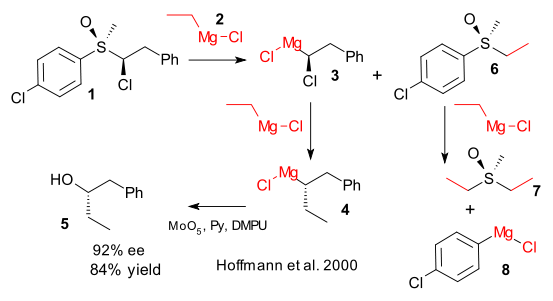 In 2000 Hoffmann et al demonstrated the synthesis and reactivity of a chiral Grignard reagent. These compounds can not ordinarily be made from the standard reaction of an (chiral) halide with magnesium metal because the stereogenic carbon atom next to magnesium at one point is a free radical with accompanying racemisation.
In 2000 Hoffmann et al demonstrated the synthesis and reactivity of a chiral Grignard reagent. These compounds can not ordinarily be made from the standard reaction of an (chiral) halide with magnesium metal because the stereogenic carbon atom next to magnesium at one point is a free radical with accompanying racemisation.
In the 2000 experiment the chiral Grignard was therefore prepared in a rather outlandisch reaction between a chiral sulfoxide (1) and three (!) equivalents of ethyl magnesiumchloride (2). The first one in an exchange reaction to intermediate 3, the second one in a substitution reaction to form the actual chiral Grignard 4 and the third one by necessity in a reaction with the sulfoxide byproduct 6 to ultimately form diethyl sulfoxide 7 and aryl magnesium chloride 8. Is there a better way?
Judging from a recent publication in Organic Letters, Chen/Fu and Xu think there is. They argue that an alternative pathway to didical formation exists by direct insertion of Mg into the halide C-X bond. In computer simulations (DFT) they reacted a hypothetical Mg4 cluster with methylbromide. Predictably the radical pathway has a much lower activation energy (with in the TS one radical delocalized over the entire Mg cluster) than any of the nonradical pathways.
But what will happen if the cluster size is increased?. Chen/Fu/Xu found that Mg9 has two nonequivalent types of Mg atoms A and B. In FMO analys the nonradical pathway proceeds by electron donation of a high energy MgA (3s) HOMO with the empty sigma orbital of the C-X halide lone pair LUMO (the halide is the electrophile). The MgA atoms are those exposed at corners or edges (defects) with lower coordination than the bulk Mg atoms. When the cluster size is increased the HOMO energy is raised favorably again as is evident from decreasing IP but the amount of defects decreases (The article only provides HOMO/LUMO data for Mg9 so direct comparison unfortunately is not possible). Regular bulk magnesium has even a much lower IP but no defects all so clearly a trade-off exists. The authors think the well-known Rieke magnesium should present the best of two worlds. Together with their choice of chlorine for the halide they feel a practical chiral Grignard is within reach. If only they could find someone to do the dirty laboratory bench work.......
