Target: taxadiene
10 December 2011 - En route to Taxol
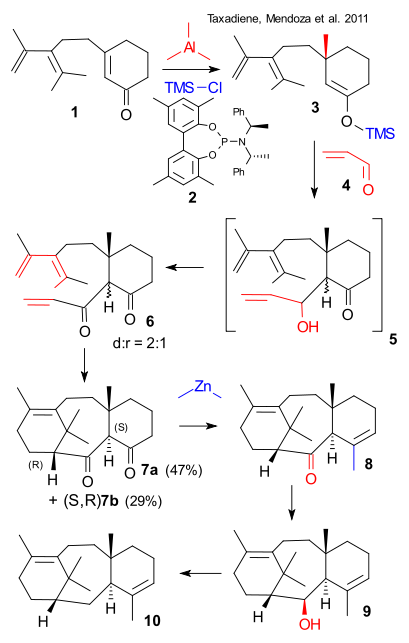 Target: (+)-taxadiene
Target: (+)-taxadiene
Who: Mendoza / Ishihara / Baran
Publication: Nature Chemistry (DOI)
Relevance: possible alternative route to Paclitaxel
Strategies: Redox economy
Advantages: gram-scale, few steps
Disadvantages: poor diastereomeric control
How: starting from achiral enone 1 Copper(I)-thiophene-2-carboxylate , chiral catalyst 2, trimethylaluminum heptane / THF then chlorotrimethylsilane to silyl enol ether 3 ( asymmetric conjugate addition), acrolein 4 (20 eq.), Gd(OTf)3 (toluene, water/EtOH) to 5 Mukaiyama aldol reaction (not isolated), then Jones reagent to diketone 6 as diastereomeric mixture, boron trifluoride etherate to tricycle 7 as diastereomeric mixture (Diels-Alder reaction) , N-phenylbis(trifluoromethanesulfonimide), KHMDS, THF to the enol triflate, then tetrakis, dimethylzinc to ketone 8 (Negishi coupling), then LiAlH4 / Et2O to alcohol 9, then KH / THF, then AcCl to acetate, then sodium, HMPA and tBuOK to taxadiene 10
Main competition: biosynthetic taxadiene by Escherichia coli starting from glucose ,Stephanopoulos 2010 ( DOI)
