Target: Molnupiravir
08 October 2021 - Covids
 There are a lot of crazies out there who will refuse covid vaccinations but will say yes to any anti-covid pill rumored to work because Facebook told them or some orange ex-president. They make it sound they take a principled decision against the vaccine (the sanctity of the human body for example) but I am suspecting they are simply afraid of needles. Several bogus anti-covid pills have been making the rounds in the media with Ivermectin the last one.
There are a lot of crazies out there who will refuse covid vaccinations but will say yes to any anti-covid pill rumored to work because Facebook told them or some orange ex-president. They make it sound they take a principled decision against the vaccine (the sanctity of the human body for example) but I am suspecting they are simply afraid of needles. Several bogus anti-covid pills have been making the rounds in the media with Ivermectin the last one.
The new antiviral drug Molnupiravir is a surprise because yes it is a pill that you can take to combat covid19 and also because it actually works. Emory University holds the original patent (2018) but Merck & Co. is currently responsible for the product development. Last week this company announced that in a clinical trial the risk of hospitalization or death was found to be reduced by 50% compared to a placebo group. A medication I guess ideally suited for patients where the vaccine did not work or for people without access to the vaccine.
The biochemistry? Yaniv Ehrlich on Twitter explains it best. The new compound resembles the RNA nucleotide cytidine but whereas cytidine forms a strong hydrogen network with nucleotide partner guanosine (the famous base-pairs) , the Molnupiravir molecule will want to pair with adenosine instead and thus create an error in a RNA chain. This antiviral strategy is nothing new, see for example Remdesivir blogged about earlier last year but Molnupiravir executes this strategy very well.
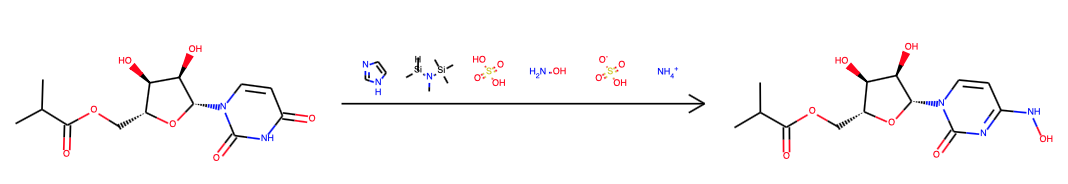
In the original Emory patent (WO2019113462) the starting material is uridine, not incidentally one of the 5 standard nucleosides. The synthetic sequence is dihydroxyl protection with acetone and sulfuric acid, acylation with Ethyl 2-(2-methoxyphenoxy)-2-methylpropanoate and DMAP/triethylamine followed by imidazole addition and finally hydroxyl amine addition. Yields were not specified, final step required chromatography.
The Merck team published a new synthesis in the end of 2020 (DOI). They dismiss uridine as a starting material on account of not being available from green chemistry and start from D-ribose instead. The other novelty is that in the first two steps out of three steps (addition of isobutyric anhydride, then uracil and then hydroxylamine sulfate) are facilitated by enzymes, no less than 5 in step 2 with a total of 16 ingredients. The work is admirable for it’s dedication to sustainability and eco-friendlyness.
The beginning of 2021 saw the publication of another Molnupiravir synthesis from a MIT / TCG Greenchem / Medicines for All team (DOI). In the key step in this synthesis cytidine is reacted with oxime ester (propan-2-ylideneamino) 2-methylpropanoate with help of an enzyme. Features: short two-step synthesis, cost-optimized, the enzyme (Novozym 435) can be recycled and the final product does not require chromatography.
By the way, the Medicines for All (M4ALL) institute is an admirable non-profit dedicated to making medications available worldwide by minimizing production costs. Made possible by the Bill or Melinda Gates Foundation.
