Ranking Oseltamivir
9 March 2009 - Green metrics
There are many different ways you can synthesise a molecular compound in the laboratory. In academics, your particular synthetic plan gets points for being elegant (a rather vague term) or for having incorporated a new break-through protocol. In industry it is all about costs which at least you can measure. But what if you want to rank synthetic plans in terms of greenness?.
John Androas of York University did some serious accounting and ranked 16 plans for the synthesis of oseltamivir (DOI) which is great because this blog has been keeping an eye on oseltamivir total synthesis, here, here and here.
A useful parameter in green metrics is the so-called reaction mass efficiency (RME) as the product of chemical yield, atom economy, the inverse of the stoichiometric factor (SF, taking into account excess reagent) and the material recovery parameter (MRP). In an ideal reaction a RME value of 1 means its four components have a value of 1 as well. Synthesis plans high in RME use few auxiliary reagents such as catalysts and as little solvent as possible. Chromatography and protective groups are nono's.
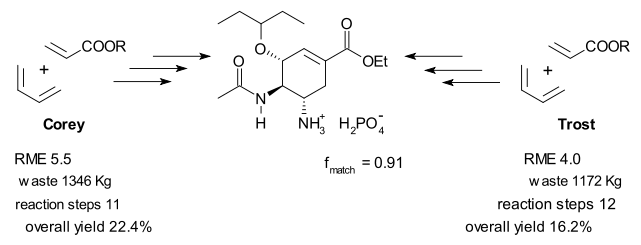
Androas reports the highest RME score out of 16 plans for Roche's shikimic acid route (RME = 11.5%). This plan also generated the least amount of waste: 94.1 kilograms per 1 mole of oseltamivir which is still an awful lot. For comparison, in all three plans developed by Shibasaki the RME value stays well below 1 with waste generated in excess of 6000 Kg. Big sacrificial reagents such as NBS and DEAD contribute to this poor performance.
Barry Trost almost got disqualified! This blog noted some time ago that Trost's commercially available lactone starting material is in fact not commercially available (perhaps if you buy it straight from his lab). Androas agrees, and for him to be able to make a sensible comparison between al plans he dug up an old 1952 plan for the lactone synthesis as a prefix to the Trost scheme. Turns out the lactone is synthesized from the same chemicals as Corey's synthesis.
Other interesting statistics from Androas' 26 page article (a 73 MB supplementary file contains a detailed description of all 16 plans) are a similarity index fmatch based on how target bonds are assembled and oxidation level profiles (a.k.a. hypsicity) for each plan. Apparently any redox reaction is bad news for atom economy and should be avoided. All Roche plan are isohypsic.
Andraos, J. (2009). Global Green Chemistry Metrics Analysis Algorithm and Spreadsheets: Evaluation of the Material Efficiency Performances of Synthesis Plans for Oseltamivir Phosphate (Tamiflu) as a Test Case Organic Process Research & Development, 13 (2), 161-185 DOI: 10.1021/op800157z
