Oxyluciferin revisited
31 January 2014 - Bugs
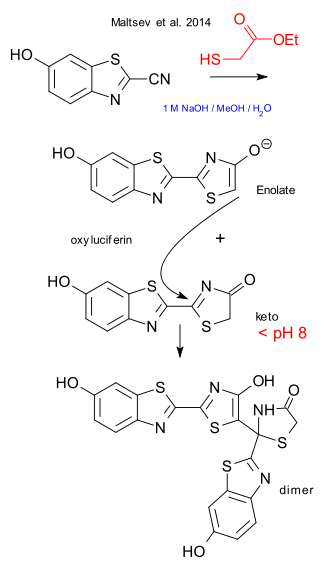 The firefly problem has already been solved in 2009 here but why is oxylucerifin so unstable? Supposedly it is impossible to have it synthesised. Maltsev et al. have been doing some reinvestigation on the synthesis of the compound from 2-cyano-6-hydroxybenzothiazole and ethyl 2-mercaptoacetate in alkaline methanol / water and have identified the culprit (DOI). With an insufficient amount of base present, the reaction product oxyluciferin is present both as the enol and keto form and both products can dimerise in a Mannich reaction. This dimer was extensively analysed via LC-HRMS and crystallography. A revised synthetic protocol for the synthesis of oxyluciferin now demands the right amount of base and low temperatures. Column chromatography is possible provided small amount of acetic acid is added to the eluent.
The firefly problem has already been solved in 2009 here but why is oxylucerifin so unstable? Supposedly it is impossible to have it synthesised. Maltsev et al. have been doing some reinvestigation on the synthesis of the compound from 2-cyano-6-hydroxybenzothiazole and ethyl 2-mercaptoacetate in alkaline methanol / water and have identified the culprit (DOI). With an insufficient amount of base present, the reaction product oxyluciferin is present both as the enol and keto form and both products can dimerise in a Mannich reaction. This dimer was extensively analysed via LC-HRMS and crystallography. A revised synthetic protocol for the synthesis of oxyluciferin now demands the right amount of base and low temperatures. Column chromatography is possible provided small amount of acetic acid is added to the eluent.
