Novel alkene aminocarboxylation
03 August 2022 - Go Green
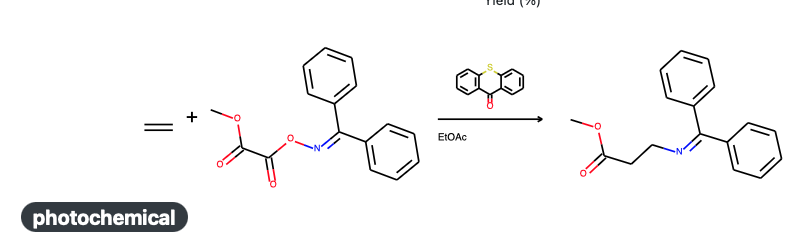 The laboratory of Frank Glorius is reporting a new way to synthesize beta amino acids (Tan, G., Das, M., Keum, H. et al. DOI). In this type of amino acid the amino group and carboxylic acid group are separated by two methylene units. Beta alanine is a very simple one (no stereocenters!) and also a naturally occurring representative. Synthesis of this type of amino acids is an active area of research and the Glorius group have been digging further into the utilization of alkenes as feed stock.
The laboratory of Frank Glorius is reporting a new way to synthesize beta amino acids (Tan, G., Das, M., Keum, H. et al. DOI). In this type of amino acid the amino group and carboxylic acid group are separated by two methylene units. Beta alanine is a very simple one (no stereocenters!) and also a naturally occurring representative. Synthesis of this type of amino acids is an active area of research and the Glorius group have been digging further into the utilization of alkenes as feed stock.
They have come up with a photochemical aminocarboxylation of simple alkenes with ethylene as prime example. An oxime oxalate ester is providing the amine and carboxylic acid and is the adduct of benzophenone oxime and methyl 2-chloro-2-oxoacetate. The photosensitizer is a simple organic compound called thioxanthone. After an acid hydrolysis step the end product is the hydrochloride acid salt of beta alanine.
In the article a reaction mechanism is proposed where the photosensitizer transfers it’s triplet state to the oxalate which then fragments over the N-O bond into an ester and an iminyl radical pair with liberation of carbon dioxide. Both fragments then add over the alkene. The article refers to the photochemical process as a triplet-triplet “energy transfer reaction” which is vague as in Wikipedia does not mention it? Perhaps Dexter energy transfer comes close.
Interestingly the article which was picked up from Frank Glorius' Twitter account was not pay walled but also not open-access. Recently (?) a netherworld has opened up in publishing land with the article “shared complimentary” courtesy of Springer Nature SharedIt. In this scheme, apparently any author can request a sharing link for social media! I was already getting bored with all these twitter announcements of new articles that on further clicking lead to nowhere. Downside of course is that you cannot download the article and that the pdf (it is an .epdf) is not searchable. If I want to know why the photosensitizer looks like abbreviated as "PC", a quick scan of the text on "PC" is simply not possible. Anyway, thanks Frank! Much appreciated! May more authors follow your example. On the other hand, this new .epdf format must also keep Lady Scihub wake at night? The end of pirating? Can it be hacked? Hackit?
