New in asymmetric photochemistry
22 November 2014 - Origins
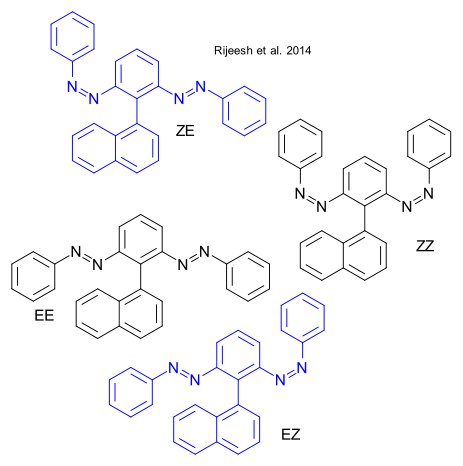 Rijeesh et al. report a novel photoinduced enantioselective reaction DOI It has been done before: in 1971 Kagan directed circularly polarized light (CLP) at a complex hydrocarbon forming a chiral helicene. The CLP handedness matched that of the helicene handedness.
Rijeesh et al. report a novel photoinduced enantioselective reaction DOI It has been done before: in 1971 Kagan directed circularly polarized light (CLP) at a complex hydrocarbon forming a chiral helicene. The CLP handedness matched that of the helicene handedness.
In the novel reaction the substrate is azobenzene EE. By CLP irradiation EE isomerizes to EZ and ZE which are each other mirror images. On further irradiation the final product ZZ is again achiral. It turns out EZ and ZE form in unequal amounts. The calculated enantiomeric excess is 0.4%.
According to the researchers EZ and ZE form in equal amounts, EZ and ZE cannot interconvert but depending on the handedness of the CLP used , one enantiomer isomerizes faster to either the final product ZZ or back to EE.
More researches appear to have rediscovered CLP as evidenced by a recent chiral coordination polymer (DOI) and a recent chiral nanowire (DOI). Background on asymmetric photochemistry here
