New in Barton decarboxylation
27 December 2016 - Organic reactions
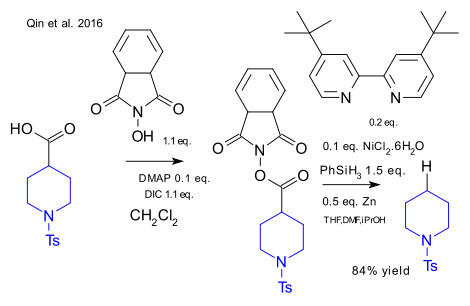 Qin et al. have reported a new take on the classic Barton decarboxylation. This organic reaction is usually associated with Barton esters that have low stability and have a terrible smell. A similar ester based on N-hydroxyphthalimide was found with equally desired electron accepting properties. In the optimized reaction this ester was reacted with elemental zinc and nickel chloride (forming an active catalyst Ni(0)) and a bipyridine ligand. Phenylsilane was the proton donor. Key advantages: low reagent costs and low water/air sensitivity, the reaction can be run as a one-pot reaction.
Qin et al. have reported a new take on the classic Barton decarboxylation. This organic reaction is usually associated with Barton esters that have low stability and have a terrible smell. A similar ester based on N-hydroxyphthalimide was found with equally desired electron accepting properties. In the optimized reaction this ester was reacted with elemental zinc and nickel chloride (forming an active catalyst Ni(0)) and a bipyridine ligand. Phenylsilane was the proton donor. Key advantages: low reagent costs and low water/air sensitivity, the reaction can be run as a one-pot reaction.
Rik
