NNNS Chemistry blog
Prevous: Cyclobutadiene X-ray structure debate continues
Next: Zinc dihydride - NHC marriage
Introducing the fluoronium ion
07 April 2013 - Orgo
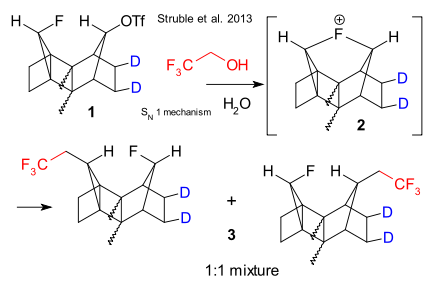 Struble et al. have presented their evidence for the existence of an organo fluoronium ion. (DOI)
Struble et al. have presented their evidence for the existence of an organo fluoronium ion. (DOI)
Onium compounds containing the other halogens are known and some can even be isolated. The high electronegativity on fluorine makes synthesis or even detection a challenge.
The experimental setup is all about compound 1 in which the triflate group can be replaced by an alcohol group via hydrolysis. But is intermediate 2 involved? Computational data suggest the symmetrical fluoronium ion is stable. The experimental crystal structure suggests the two methylene bridges repel each other. After labelling the compound and replacing water with trifluoroethanol the new product is a 1:1 mixture of 3 (NMR) , consistent with the fluoronium ion.
